Question: for second question, the choices are 1 (increase/decrease/stay the same) 2 (greater than/wqual/less than) 3 (increase/decrease/stay the same) Consider the following system at equilibrlum where
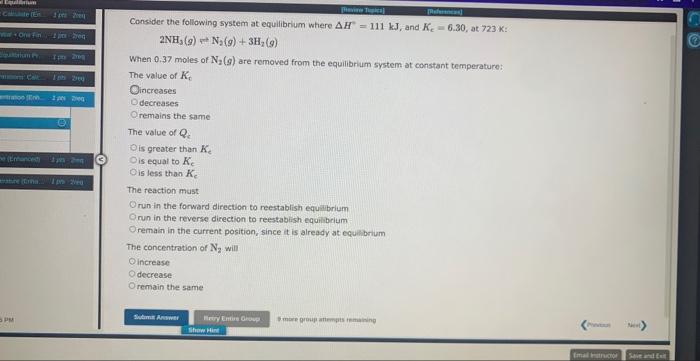
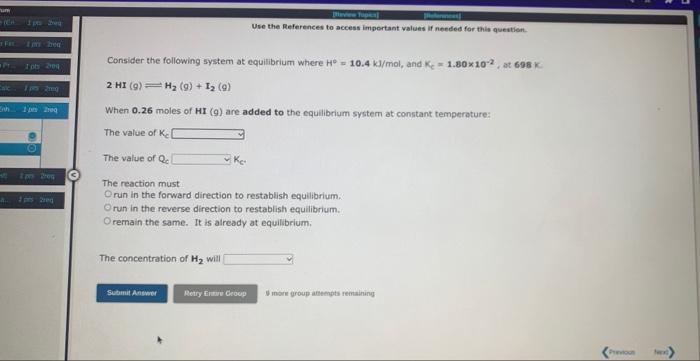 for second question, the choices are 1 (increase/decrease/stay the same) 2 (greater than/wqual/less than) 3 (increase/decrease/stay the same)
for second question, the choices are 1 (increase/decrease/stay the same) 2 (greater than/wqual/less than) 3 (increase/decrease/stay the same)Consider the following system at equilibrlum where H=111kJ, and Kc=6.30, at 723K : 2NH3(g)N2(g)+3H2(g) When 0.37 moles of N2(g) are removed from the equilibrium system at constant temperature: The value of Kc increases decreasen remains the same The value of Qc. is greater than Kc is equal to Kc is less than Kc The reaction must run in the forward direction to reestablish equalbrium nu in the reverse direction to reestabish equilibrium remain in the current position, since it is already at equilbrium The concentration of N2 will increase decrease remain the same Consider the following system at equilibrium where H=10.4kJ/mol, and K,=1.30102, at 608K 2HI(g)H2(g)+I2(g) When 0.26 moles of HI (9) are added to the equilibrium system at constant temperature: The value of K e The value of Qc The reaction must run in the forward direction to restablish equilibrium. run in the reverse direction to restablish equilibrium. remain the same. It is already at equilibrium. The concentration of H2 will 4 more group aftempts remathing
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


