Question: For the binary liquid system, A-B the following relative partial functions are listed for 1045C Calculate the relative partial entropy as well as the activity
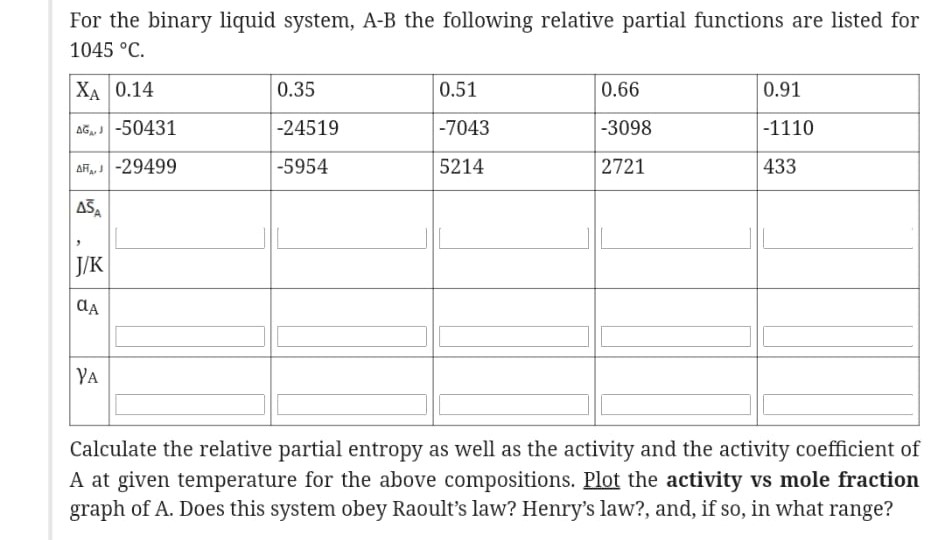
For the binary liquid system, A-B the following relative partial functions are listed for 1045C Calculate the relative partial entropy as well as the activity and the activity coefficient of A at given temperature for the above compositions. Plot the activity vs mole fraction graph of A. Does this system obey Raoult's law? Henry's law?, and, if so, in what range? For the binary liquid system, A-B the following relative partial functions are listed for 1045C Calculate the relative partial entropy as well as the activity and the activity coefficient of A at given temperature for the above compositions. Plot the activity vs mole fraction graph of A. Does this system obey Raoult's law? Henry's law?, and, if so, in what range
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


