Question: For the eutectic phase diagram shown above at the temperature given by the dotted line, the two Gibbs free energy diagrams are equally valid. Explain
For the eutectic phase diagram shown above at the temperature given by the dotted line, the two Gibbs free energy diagrams are equally valid. Explain the difference between the two and why they are both valid.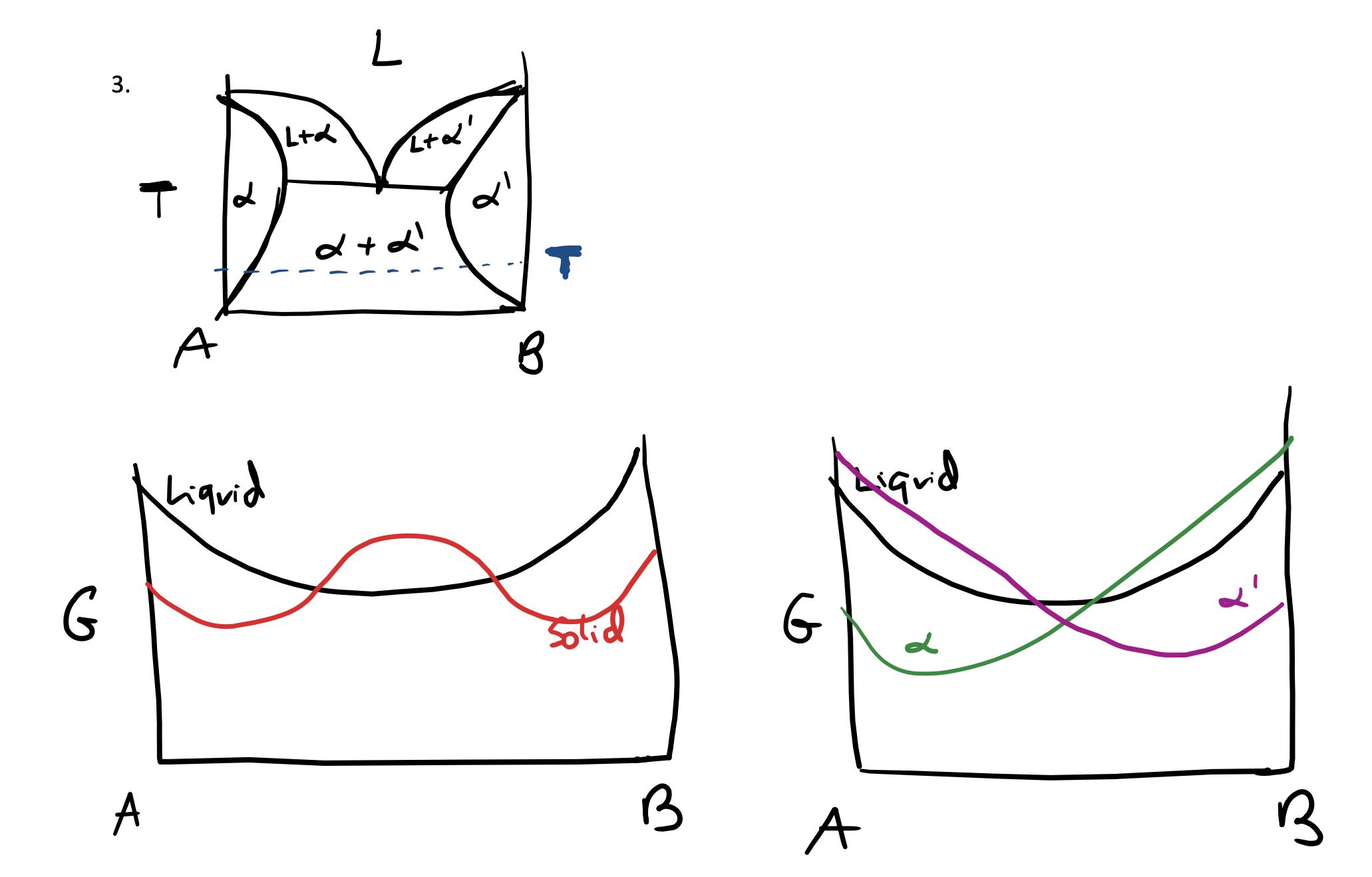
G L l+d l+d' pa 1 2 A 8 3. A chiquid + d stid B G biguid A B
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
First Order Transitions The following plot shows the Gibbs energy as a function of temperature including phase changes from solid to liquid melting an... View full answer

Get step-by-step solutions from verified subject matter experts


