Question: For the free-chlorine dose and pH range given in Problem 13-3, calculate and plot the required disinfectant contact time as a function of pH to

For the free-chlorine dose and pH range given in Problem 13-3, calculate and plot the required disinfectant contact time as a function of pH to achieve 2-log inactivation of the bacteria.
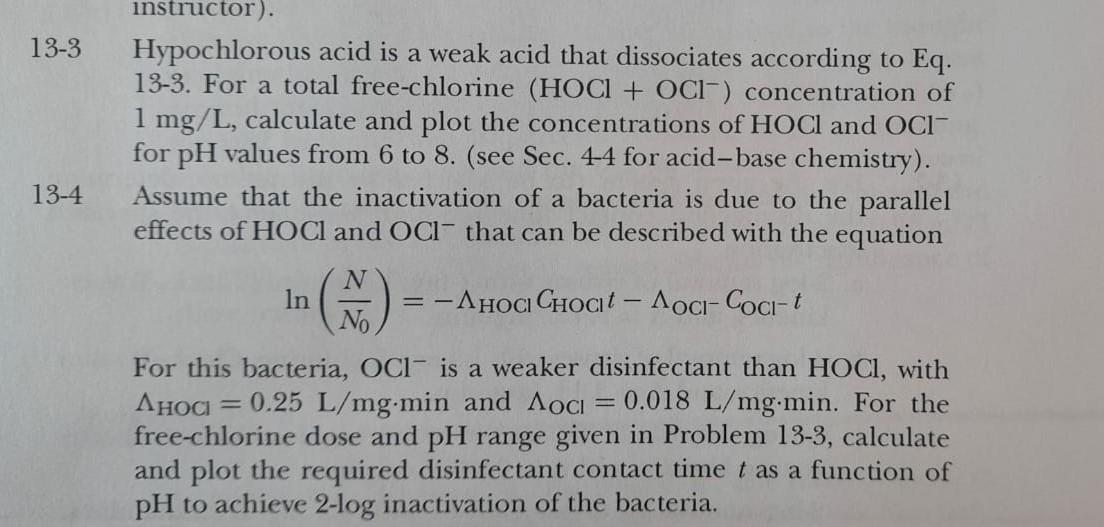
Assume that the inactivation of a bacteria is due to the parallel effects of HOCl and Ocl- that can be described with the equation N In = -AHOGI Choct - Aoci-Coci-t For this bacteria, Oci is a weaker disinfectant than HOCI, with Ahoa = 0.25 L/mg.min and Aoci = 0.018 L/mg.min. For the free-chlorine dose and pH range given in Problem 13-3, calculate and plot the required disinfectant contact time t as a function of pH to achieve 2-log inactivation of the bacteria. 13-3 13-4 instructor). Hypochlorous acid is a weak acid that dissociates according to Eq. 13-3. For a total free-chlorine (HOCI + OCl-) concentration of 1 mg/L, calculate and plot the concentrations of HOCI and OCI- for pH values from 6 to 8. (see Sec. 4-4 for acid-base chemistry). Assume that the inactivation of a bacteria is due to the parallel effects of HOCl and OCl- that can be described with the equation N In -Ahoa Choct - Aoci-Coci- t No For this bacteria, OCl is a weaker disinfectant than HOCI, with AHOCI 0.25 L/mg.min and Aoci 0.018 L/mg.min. For the free-chlorine dose and pH range given in Problem 13-3, calculate and plot the required disinfectant contact time t as a function of pH to achieve 2-log inactivation of the bacteria. - =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


