Question: For the gas phase reaction: A+B C+D The feed stream at 500 K and 2 atm flows through a plug flow reactor with an
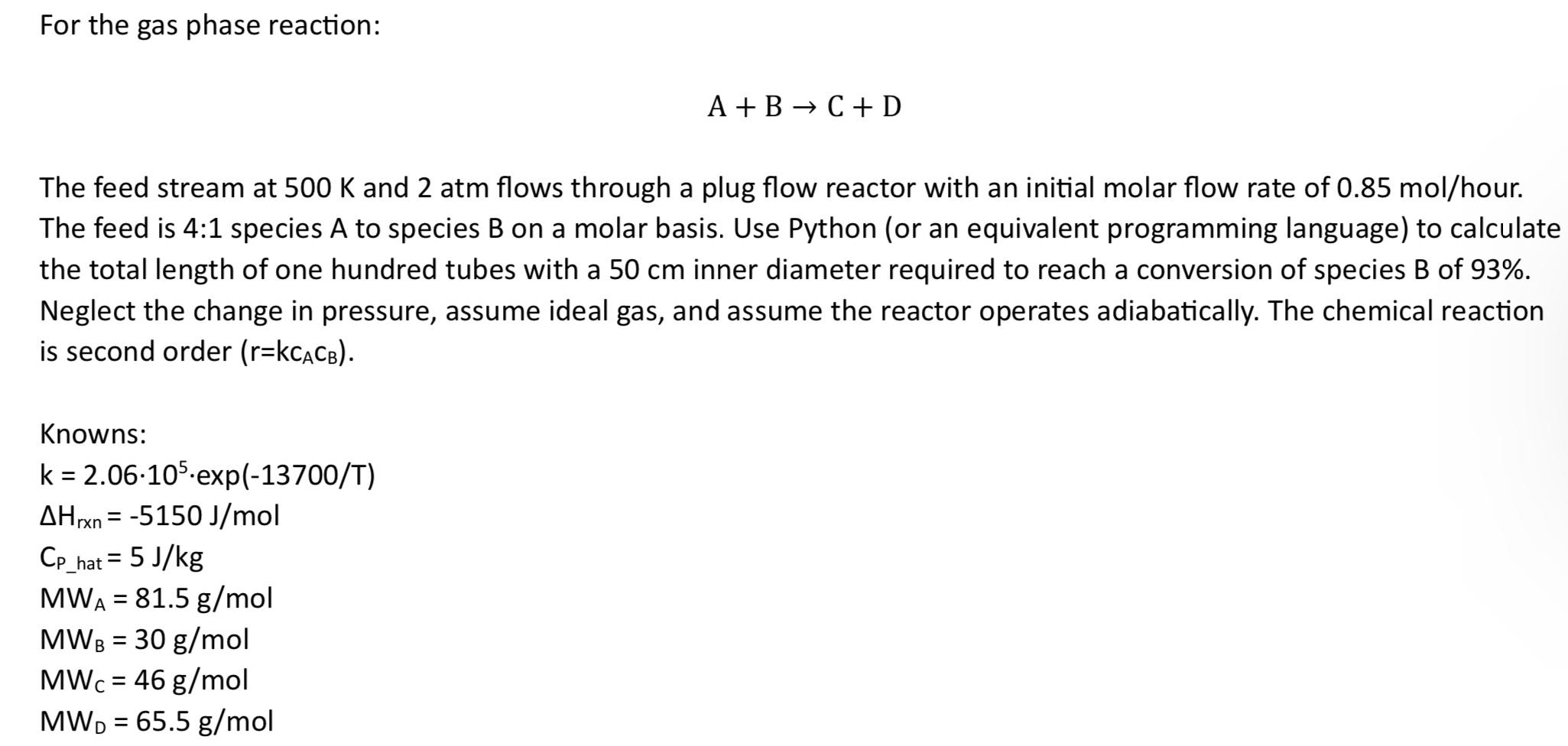
For the gas phase reaction: A+B C+D The feed stream at 500 K and 2 atm flows through a plug flow reactor with an initial molar flow rate of 0.85 mol/hour. The feed is 4:1 species A to species B on a molar basis. Use Python (or an equivalent programming language) to calculate the total length of one hundred tubes with a 50 cm inner diameter required to reach a conversion of species B of 93%. Neglect the change in pressure, assume ideal gas, and assume the reactor operates adiabatically. The chemical reaction is second order (r=KCACB). Knowns: k = 2.06.105 exp(-13700/T) AHrxn=-5150 J/mol Cp_hat= 5 J/kg MWA = 81.5 g/mol MWB = 30 g/mol MWc = 46 g/mol MWD = 65.5 g/mol
Step by Step Solution
There are 3 Steps involved in it
Solution Here is Python code that can solve this problem python Import math module import math Defin... View full answer

Get step-by-step solutions from verified subject matter experts


