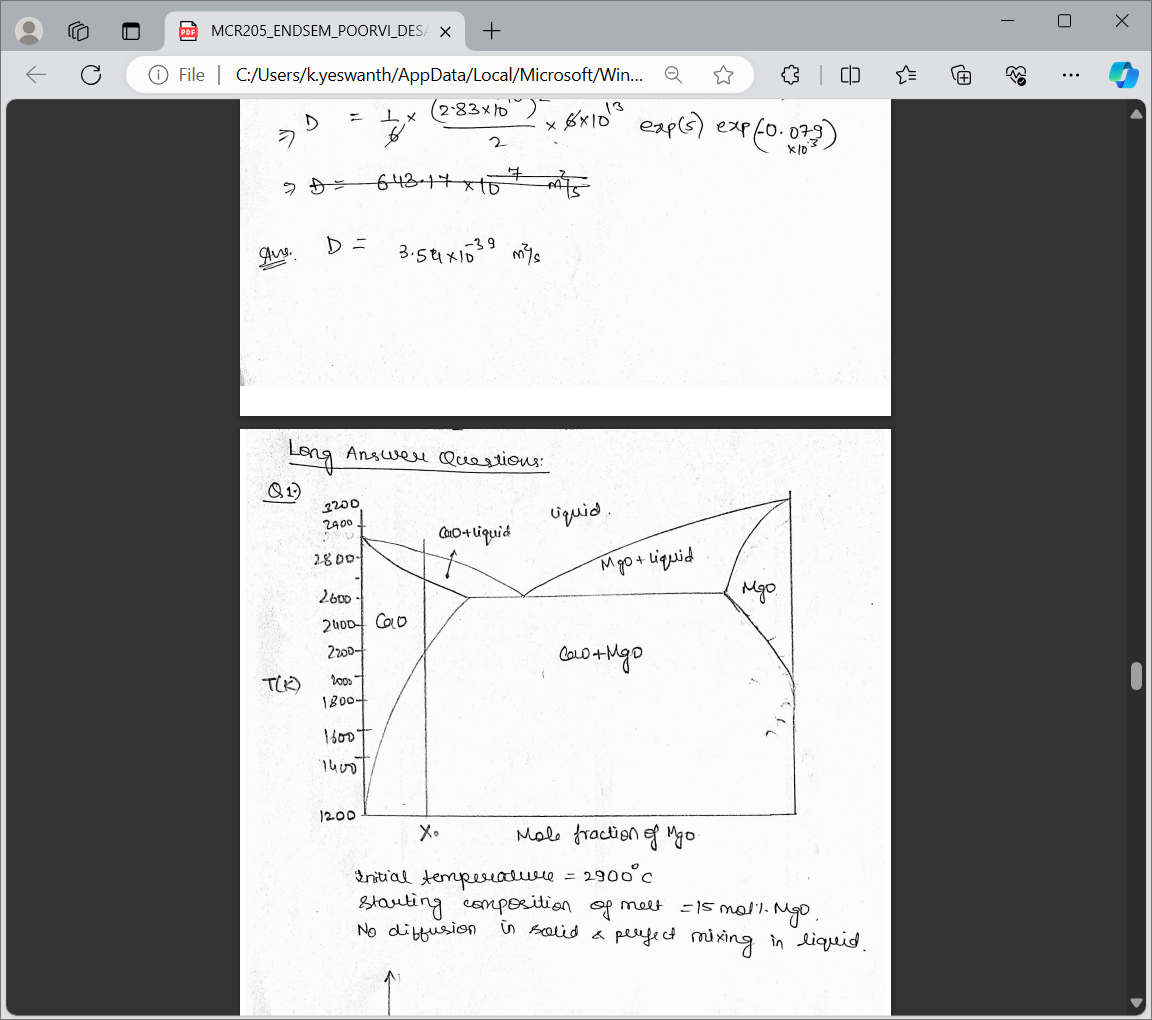
Question: For the given CaO - MgO system ( Figure 1 ) , show the schematic MgO composition profile = > D = 1 6 (
For the given CaOMgO system Figure show the schematic MgO composition profile exp
:
Ans.
Long Answer Questions:
Q
Initial temperalure
stauting composition of melt mol.Mgo.
No diffurion in solid a prefect mixing in liquid.
as a function of distance in a rod element at different stages of cooling when solidification
interface sweeps across the length. How much eutectic will form after complete
solidification? Assume the followings:
a Initial temperature is deg C starting composition of melt is mol MgO.
b Heat is extracted unidirectionally through one of the shorter wall of the rod
element.
c No diffusion in solid and perfect mixing in liquid.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


