Question: For the given reaction, explain how IR spectroscopy can be used to monitor the progress of the reaction. Select all answers that apply. H30+ .
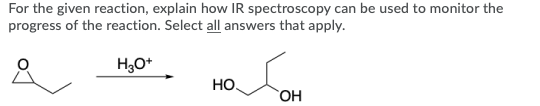
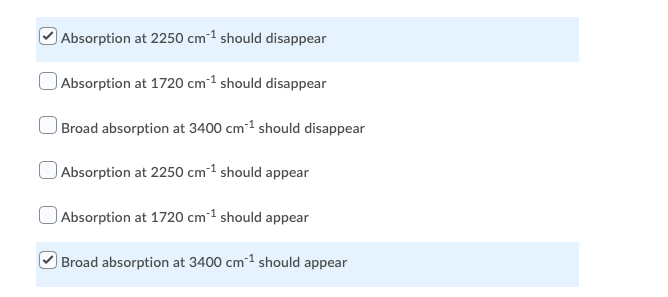
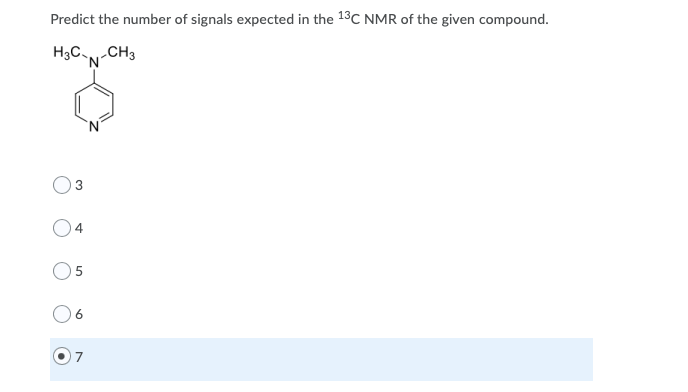
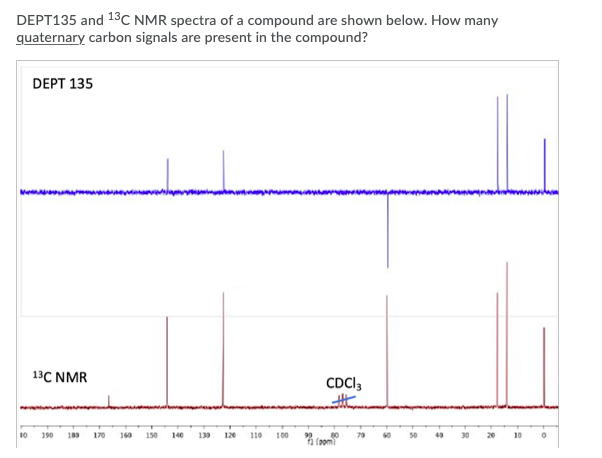
For the given reaction, explain how IR spectroscopy can be used to monitor the progress of the reaction. Select all answers that apply. H30+ . OH Absorption at 2250 cm 1 should disappear Absorption at 1720 cm-1 should disappear Broad absorption at 3400 cm 1 should disappear Absorption at 2250 cm 1 should appear Absorption at 1720 cm-1 should appear Broad absorption at 3400 cm-1 should appear Predict the number of signals expected in the 13C NMR of the given compound. H3C-N-CH3 7 DEPT135 and 13C NMR spectra of a compound are shown below. How many quaternary carbon signals are present in the compound? DEPT 135 13C NMR CDCI3 10 190 103 170 160 150 140 13 120 110 100 70 10 50 30 20 10 0 sroom
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


