Question: For the hydrogen atom and the trial function where a is a variational parameter, using atomic units, calculate the expectation values of the potential
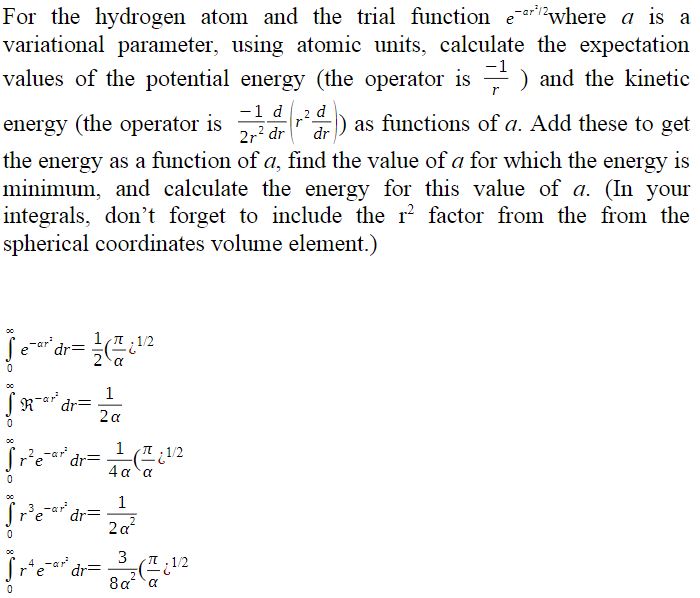
For the hydrogen atom and the trial function where a is a variational parameter, using atomic units, calculate the expectation values of the potential energy (the operator is 1) and the kinetic -1 d d energy (the operator is 14 (24) as functions of a. Add these to get dr) 2r dr the energy as a function of a, find the value of a for which the energy is minimum, and calculate the energy for this value of a. (In your integrals, don't forget to include the r factor from the from the spherical coordinates volume element.) 0 dr= Rardr= 1 1/2 2 a 1 2 a [Pe=112 0 dr= 0 -ar dr= rtear dr= 0 4 a a 1 2a 3 2 1/2 8a a
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


