Question: For the next 2 problems, consider the generic chemical equation, where the initial concentration of each component is: [A]0=3.0M,[B]0=0.85M,[C]0=1.0M(T=298K) 3A(aq)2B(aq)+C(aq) 5) What is the value
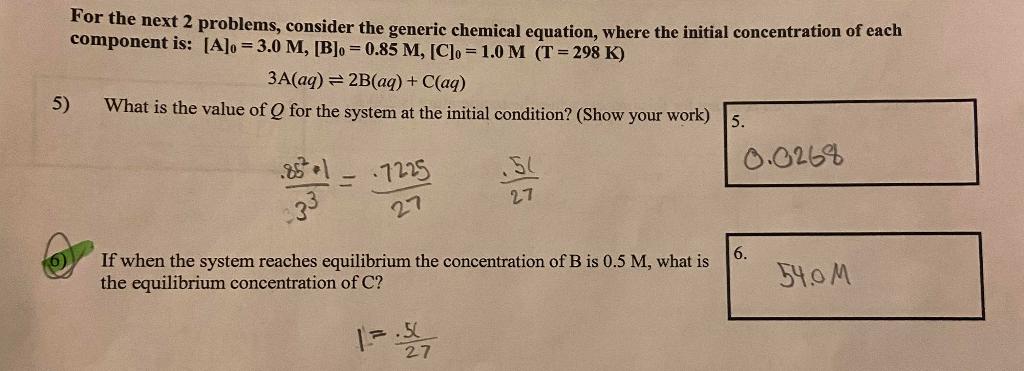
For the next 2 problems, consider the generic chemical equation, where the initial concentration of each component is: [A]0=3.0M,[B]0=0.85M,[C]0=1.0M(T=298K) 3A(aq)2B(aq)+C(aq) 5) What is the value of Q for the system at the initial condition? (Show your work) 33.852+1=27.722527.51 If when the system reaches equilibrium the concentration of B is 0.5M, what is the equilibrium concentration of C ? 1=27.5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


