Question: For the reaction A - products, concentration and time data were collected. Enter these data into the graphing tool to determine the reaction order. 1
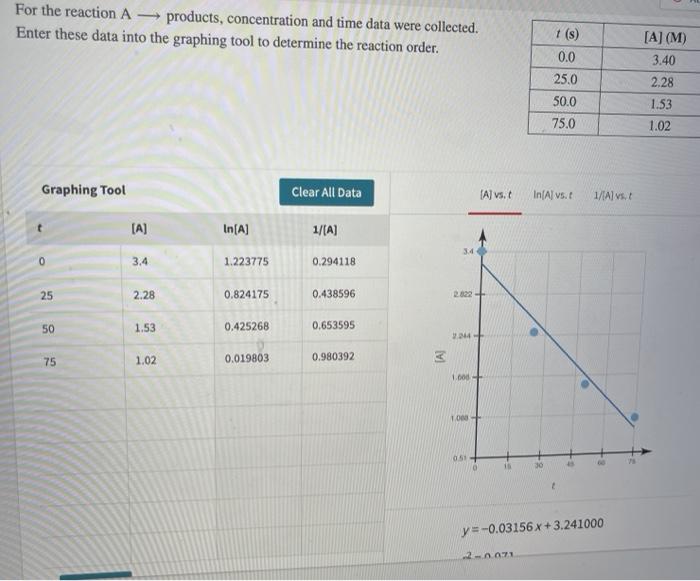
![order. 1 (s) 0.0 25.0 50.0 75.0 [A] (M) 3.40 2.28 1.53](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f91aca97547_35466f91aca3f971.jpg)
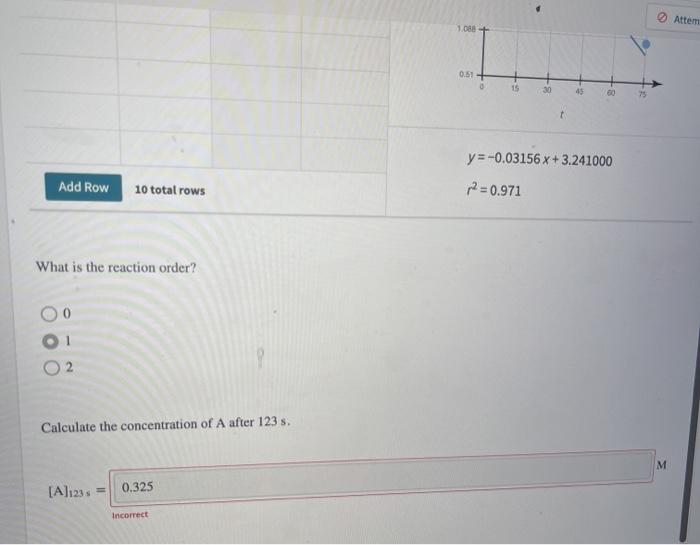
For the reaction A - products, concentration and time data were collected. Enter these data into the graphing tool to determine the reaction order. 1 (s) 0.0 25.0 50.0 75.0 [A] (M) 3.40 2.28 1.53 1.02 Graphing Tool Clear All Data Al vs. In(Al vs. 1/1A) vs. (A) In(A) 1/(A) 3.4 0 3.4 1.223775 0.294118 25 2.28 0.824175 0.438596 2.822+ 50 1.53 0.425268 0.653595 NE 75 1.02 0.019803 0.980392 IA 1.000 1.000 M 3 0 y=-0.03156x+3.241000 2071 Attem 1088+ 0.51 0 15 30 0 + y=-0.03156 x + 3.241000 Add Row 10 total rows p=0.971 What is the reaction order? 2 Calculate the concentration of A after 123 s. M [A]123 0.325 Incorrect Consider the first-order reaction described by the equation H H H.C ECH, HC- CH H H Cyclopropane Propene At a certain temperature, the rate constant for this reaction is 5.60 x 10-4-!. Calculate the half-life of cyclopropane at this temperature. #12 = 1237.5 Given an initial cyclopropane concentration of 0.00510 M, calculate the concentration of cyclopropane that remains after 2.30 hours. M concentration: 4.27 x10-5 incorrect
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


