Question: For the reaction below, N2O5 is a first order reactant. At 293K the rate constant is 2.010551. Predict the concentration of N2O5 after a sample
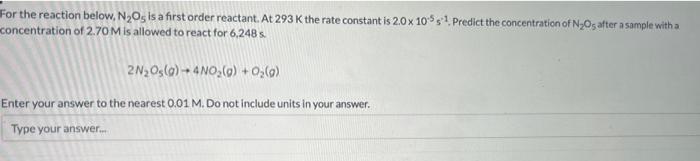
For the reaction below, N2O5 is a first order reactant. At 293K the rate constant is 2.010551. Predict the concentration of N2O5 after a sample with a concentration of 2.70M is allowed to react for 6,248s. 2N2O5(g)+4NO2(g)+O2(g) Enter your answer to the nearest 0.01M. Do not include units in your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


