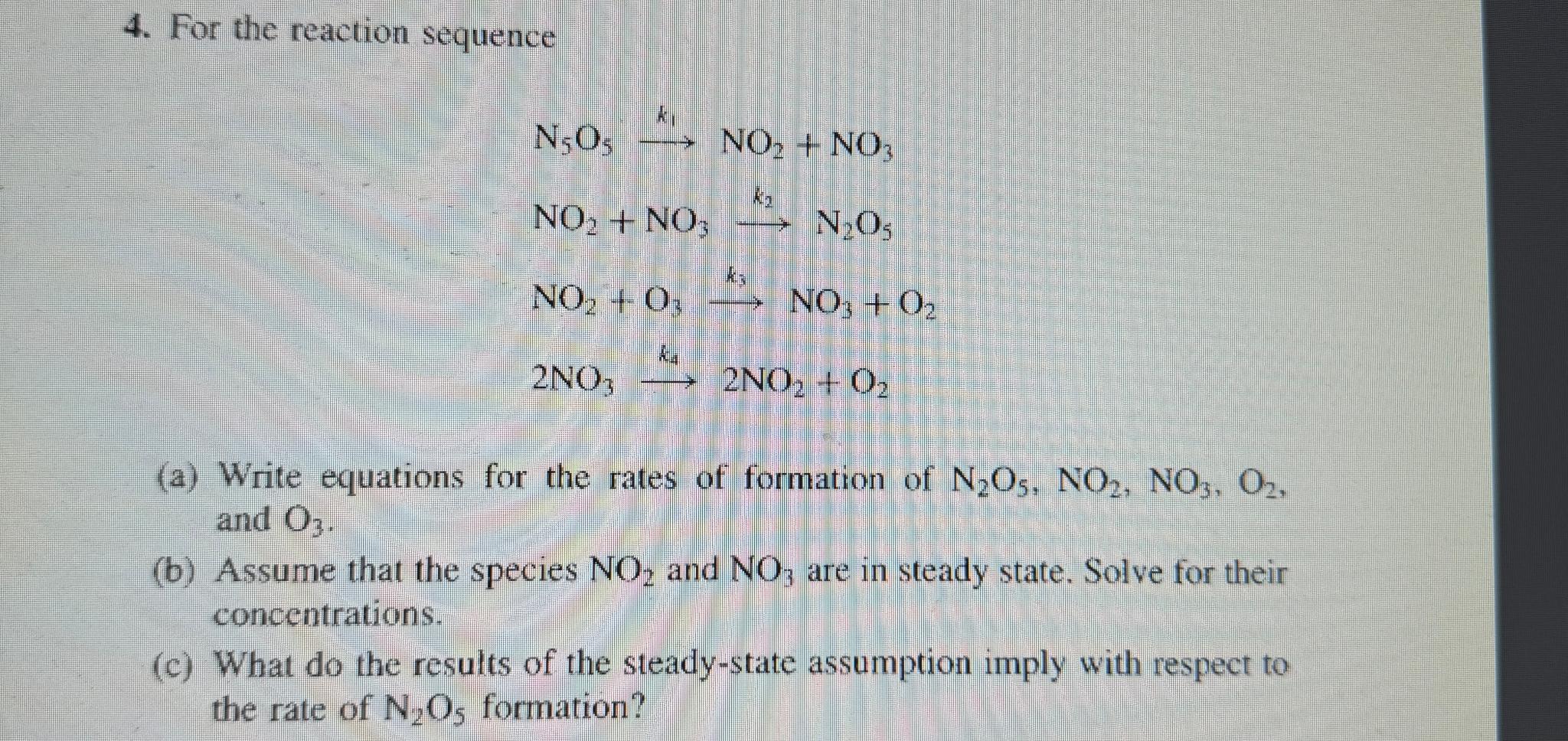
Question: For the reaction sequence N 5 O 5 k 1 N O 2 + N O 3 N O 2 + N O 3 k
For the reaction sequence
a Write equations for the rates of formation of and
b Assume that the species and are in steady state. Solve for their concentrations.
c What do the results of the steadystate assumption imply with respect to the rate of formation?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


