Question: For the second reaction in Table 4.1, determine the forward reaction rate constant at a temperature of 1000 K. Table 4.1 Recommended rate coefficients for
For the second reaction in Table 4.1, determine the forward reaction rate constant at a temperature of 1000 K.
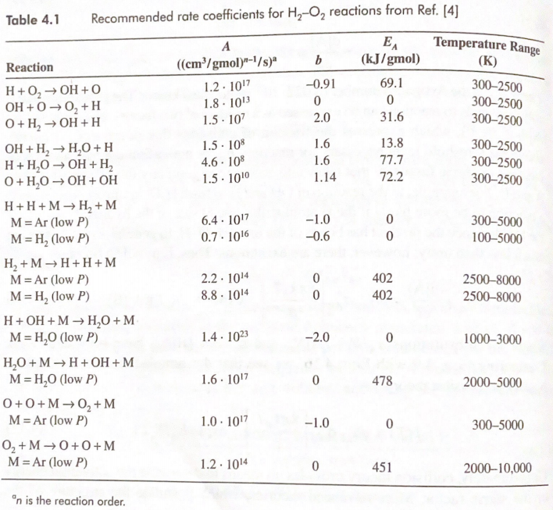
Table 4.1 Recommended rate coefficients for H, O, reactions from Ref. (4) b Reaction A ((cm/gmol)-/s) 1.2.1017 1.8. 1013 1.5. 107 1.5 - 10% 4.6. 108 1.5.100 -0.91 0 2.0 EA (kJ/gmol) 69.1 0 31.6 Temperature Range (K) 300-2500 300-2500 300-2500 300-2500 300-2500 300-2500 1.6 1.6 1.14 13.8 77.7 72.2 6.4. 1017 0.7.1016 -1.0 -0.6 0 0 300-5000 100-5000 H+ O2 OH+O OH +0 -0+H O + H2 OH+H OH + H, H,O+H H+H2O OH+H, O+H,0 OH + OH H+H+ MH+M M = Ar (low P) M=H, (low P) H, +MH+H+M M=Ar (low P) M=H, (low P) H+OH+MH0+M M = H,0 (low P) H,O+MH+OH+M M=H,0 (low P) 0+0+ MO+M M = Ar (low P) 0,+MO+O+M M = Ar (low P) 2.2.10 8.8. 101 0 0 402 402 2500-8000 2500-8000 1.4. 1023 -2.0 0 1000-3000 1.6. 1017 0 478 2000-5000 1.0.1017 - 1.0 0 300-5000 1.2. 1014 0 451 2000-10,000 on is the reaction order
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


