Question: For the simple cubic crystal structure (having the hard-sphere unit cell shown below) and having an atomic radius of R, there are two types of
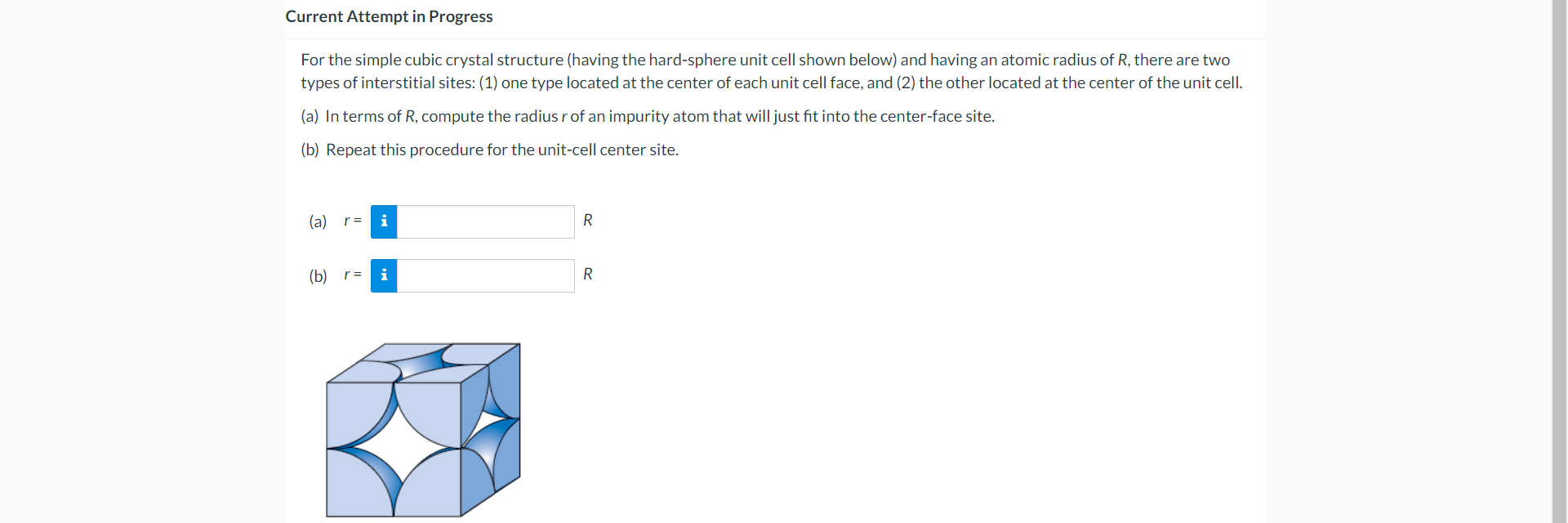
For the simple cubic crystal structure (having the hard-sphere unit cell shown below) and having an atomic radius of R, there are two types of interstitial sites: (1) one type located at the center of each unit cell face, and (2) the other located at the center of the unit cell. (a) In terms of R, compute the radius r of an impurity atom that will just fit into the center-face site. (b) Repeat this procedure for the unit-cell center site
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


