Question: For the system n - pentane ( 1 ) - n - heptane ( 2 ) , the vapour pressures are given by Page 2
For the system pentane heptane the vapour pressures are given by
Page of
CHT
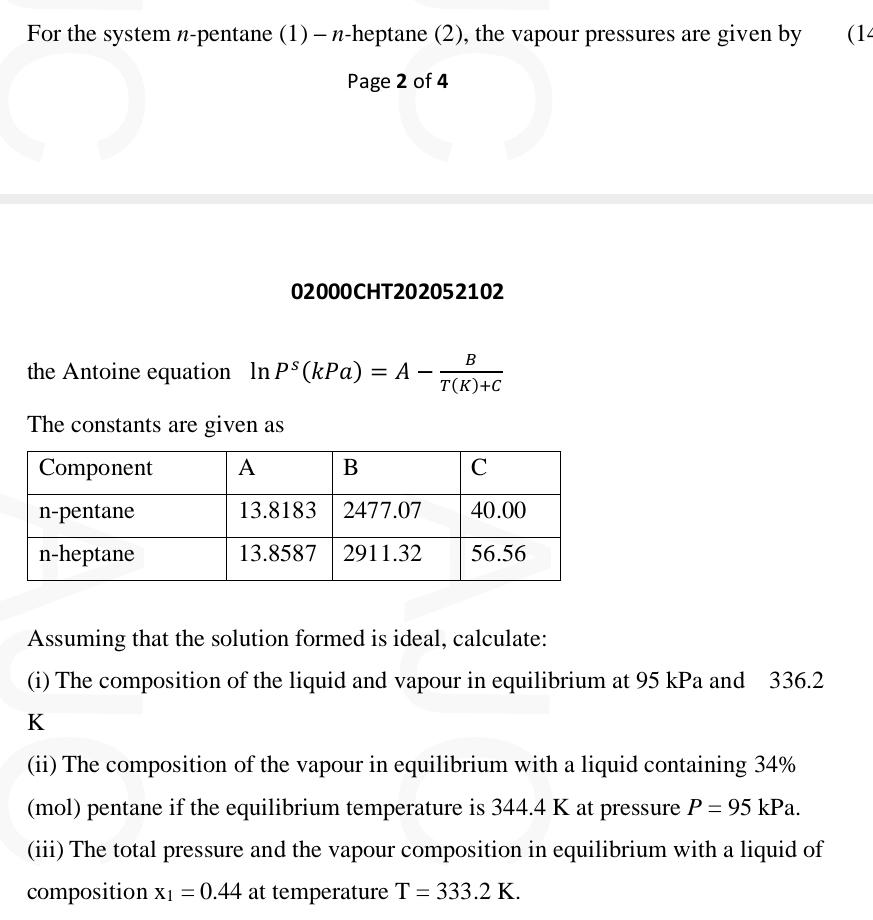
the Antoine equation
The constants are given as
tableComponentABCnpentane,nheptane,
Assuming that the solution formed is ideal, calculate:
i The composition of the liquid and vapour in equilibrium at kPa and
ii The composition of the vapour in equilibrium with a liquid containing mol pentane if the equilibrium temperature is at pressure kPa.
iii The total pressure and the vapour composition in equilibrium with a liquid of composition at temperature
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


