Question: For the unknowns, you are given approximate concentrations. Utilize this information as well as the C1V1=C2V2 equation to calculate the volumes of concentrated sample and
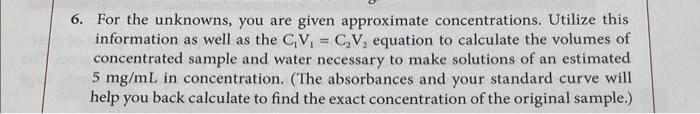

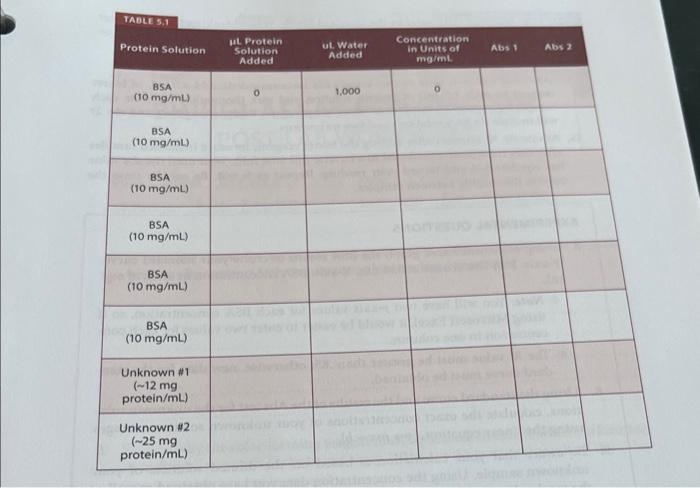
For the unknowns, you are given approximate concentrations. Utilize this information as well as the C1V1=C2V2 equation to calculate the volumes of concentrated sample and water necessary to make solutions of an estimated 5mg/mL in concentration. (The absorbances and your standard curve will help you back calculate to find the exact concentration of the original sample.) Biuret reagent. This reagent was prepared by first boiling 1.5L of distilled water to decompose any residual protein in the water. The following reagents were then individually dissolved in small amounts of the cooled water: 1.5gCuSO45H2O,6.0g sodium potassium tartrate, and 30gCO2-free NaOH. The dissolved reagents were mixed and diluted to 1,000mL with the cooled water. Standard protein solution ( 10mg/mL bovine serum albumin (BSA)) Solution with unknown protein concentration \#1 ( 12mgprotein/1,000L) Solution with unknown protein concentration \#2 ( 25mg protein/1,000 L)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


