Question: for these 2 problems, when solving for WORK. Why one requires mass to be multiplied in the work equation and the other problem does not

for these 2 problems, when solving for WORK. Why one requires mass to be multiplied in the work equation and the other problem does not require that ? please give me the explanation to that. how can I tell whether I have to do that or no?
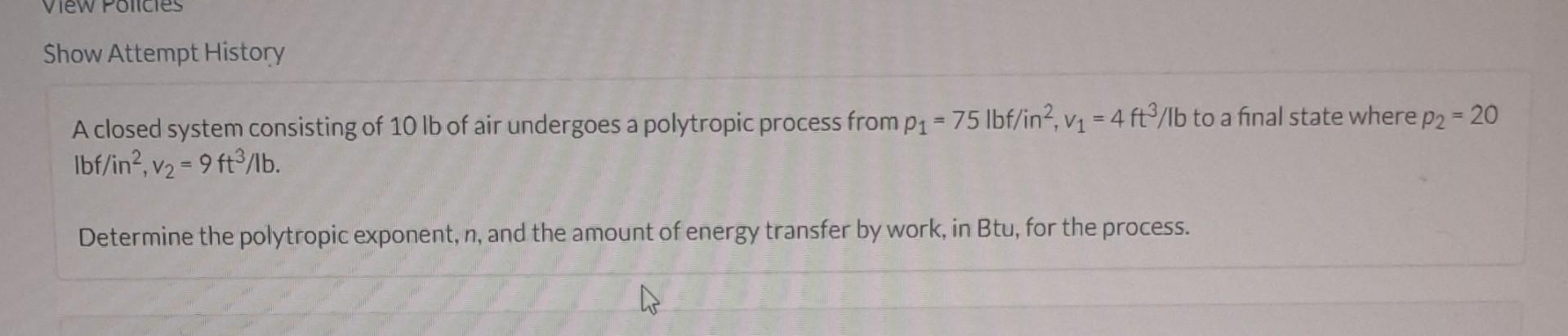
A closed system consisting of 10lb of air undergoes a polytropic process from p1=75lbf/in2,v1=4ft3/lb to a final state where p2=20 lbf/in2,v2=9ft3/lb. Determine the polytropic exponent, n, and the amount of energy transfer by work, in Btu, for the process. Steam in a piston-cylinder assembly undergoes a polytropic process, with n=2, from an initial state where V1=4.38600ft3,p1=350 Ibf/in2, and u1=1322.4Btu/lb to a final state where u2=1036.0Btu/lb and v2=3.393ft3/lb. The mass of the steam is 2.5lb. Changes in kinetic and potential energy can be neglected. Determine the change in volume, in ft3, the energy transfer by work, in Btu, and the energy transfer by heat, in Btu
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


