Question: For these next 3 questions, answer whether there are more H3O+(ieH+),OH-ions, or are they equal. 3. Click and drag the green circle into water. a)
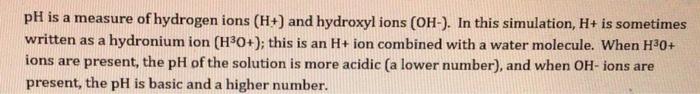
For these next 3 questions, answer whether there are more H3O+(ieH+),OH-ions, or are they equal. 3. Click and drag the green circle into water. a) What happens to the ions? (more H3O+, more OH, or are they equal? b) What happens to the ions in drain cleaner? c) What happens to the ions in vomit? 4. According to what we have learned in this simulation, why is water neutral? pH is a measure of hydrogen ions (H+t and hydroxyl ions (OH). In this simulation, H+ is sometimes written as a hydronium ion (H3O+); this is an H+ ion combined with a water molecule. When H30+ ions are present, the pH of the solution is more acidic (a lower number), and when OH - ions are present, the pH is basic and a higher number
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


