Question: For this experiment, use the table below (Table 1) to help you prepare your mixiures. Stock solution Concentrations concentration of KI I concentration of Na2S2O3OO
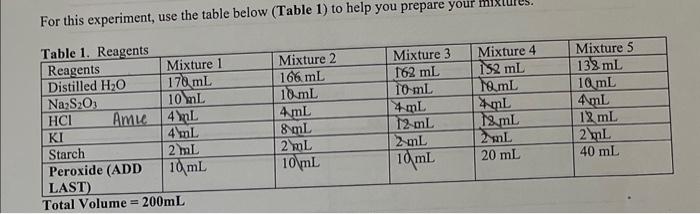

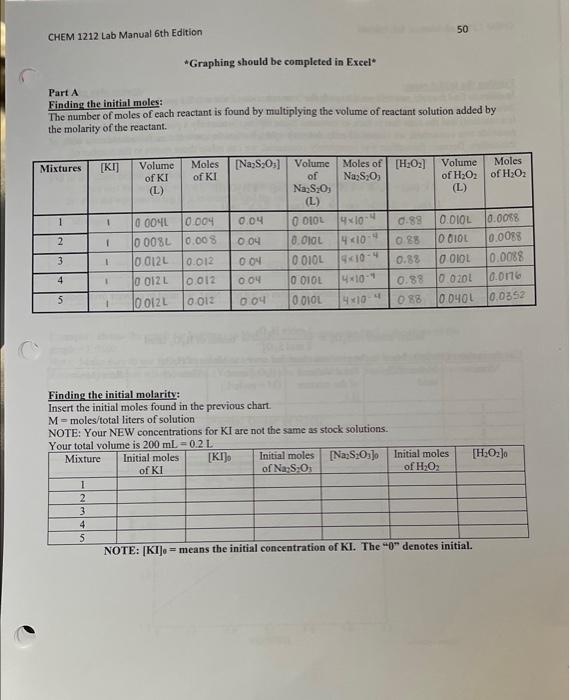
For this experiment, use the table below (Table 1) to help you prepare your mixiures. Stock solution Concentrations concentration of KI I concentration of Na2S2O3OO concentration of H2O20.88 Part A: Effect of Concentration Room Temperature: Part R. Riffaet nf Tamanamatuma Room temp: 19.6C 1) 22.3C 2) 20.9C 3) 21.6C 4) 21.1C 5) 21.9C "Graphing should be completed in Exeel" Part A Finding the initial moles: The number of moles of each reactant is found by multiplying the volume of reactant solution added by the molarity of the reactant. Finding the initial molarity: Insert the initial moles found in the previous chart. M= moles / total liters of solution NOTE: Your NEW concentrations for KI are not the same as stock solutions. NOTE: [KI]0= means the initial concentration of KI. The "" denotes mitial
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


