Question: For this heterogeneous system 2 A(aq) + 3 B(g) + C(1) = 2D(s) + 3 E(g) = the concentrations and pressures at equilibrium are [A]
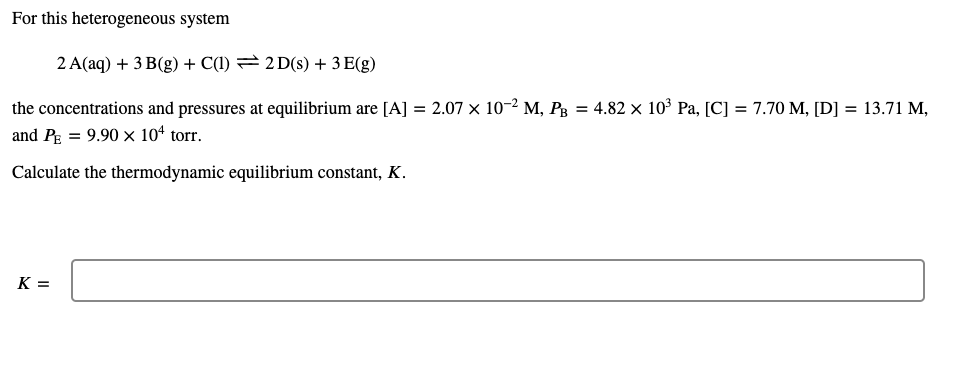
For this heterogeneous system 2 A(aq) + 3 B(g) + C(1) = 2D(s) + 3 E(g) = the concentrations and pressures at equilibrium are [A] = 2.07 x 10-2 M, PB = 4.82 x 10? Pa, [C] = 7.70 M, [D] = 13.71 M, and PE = 9.90 x 104 torr. Calculate the thermodynamic equilibrium constant, K. K=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


