Question: For this problem, we are going to use the plot (and only the plot) shown in problem 7.54 [Koretsky, 2nd edition], but nothing else from
![only the plot) shown in problem 7.54 [Koretsky, 2nd edition], but nothing](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f6cf2e0e39d_92566f6cf2d99f40.jpg)
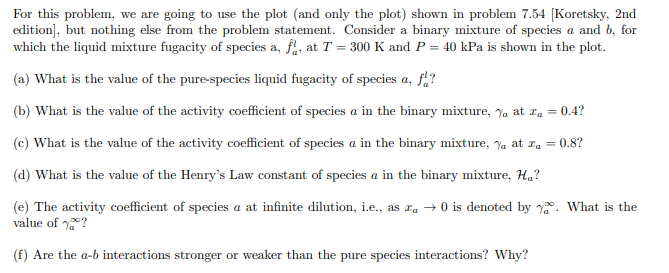
For this problem, we are going to use the plot (and only the plot) shown in problem 7.54 [Koretsky, 2nd edition], but nothing else from the problem statement. Consider a binary mixture of species a and b, for which the liquid mixture fugacity of species a, f, at T = 300 K and P = 40 kPa is shown in the plot. (a) What is the value of the pure-species liquid fugacity of species a, f? (b) What is the value of the activity coefficient of species a in the binary mixture, % at za = 0.4? (c) What is the value of the activity coefficient of species a in the binary mixture, % at a = 0.8? (d) What is the value of the Henry's Law constant of species a in the binary mixture, Ha? (e) The activity coefficient of species a at infinite dilution, i.e., as a 0 is denoted by. What is the value of ? (f) Are the a-b interactions stronger or weaker than the pure species interactions? Why? (kPa) 40 38 36 34 32 30 28 26 24 22 20 18 16 14 12 10 T= 300 K P= 40 kPa 8 6 4 2 0 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 Xa
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


