Question: For this question, use steam tables in Schroeder's chapter 4, available here: T P H water Hsteam Swater Ssteam (C) (bar) (kJ) (kJ) (KJ/K) (KJ/K)
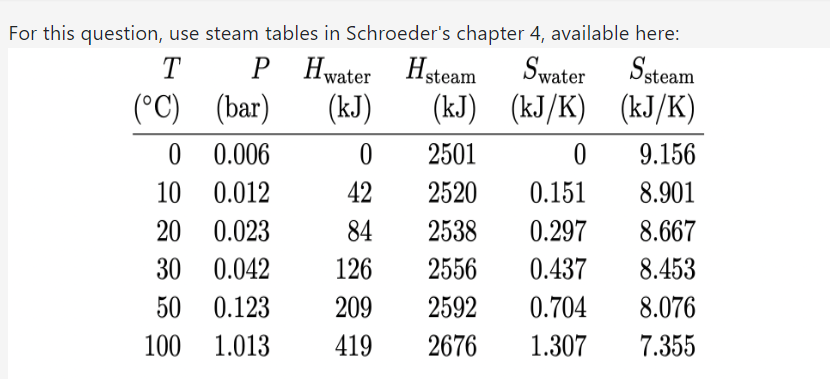
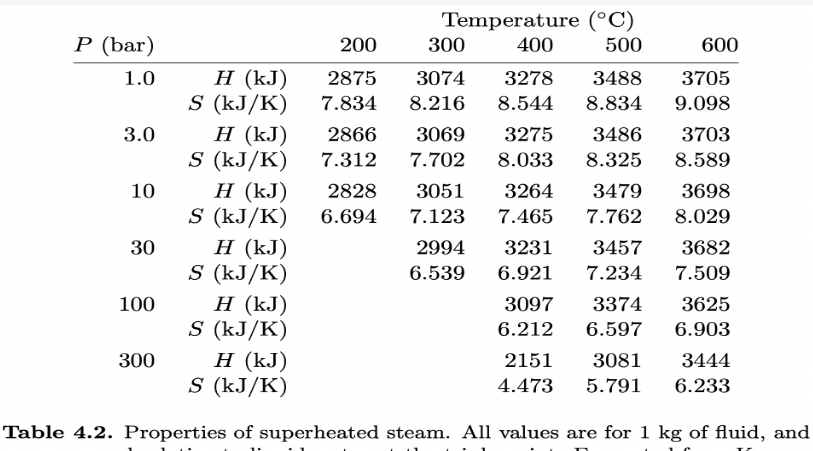


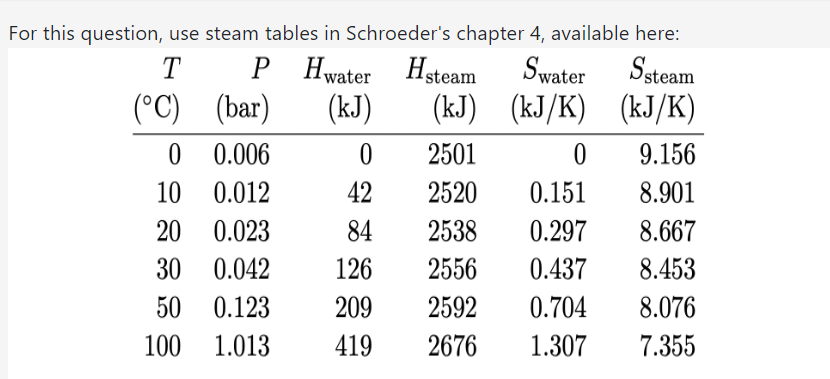
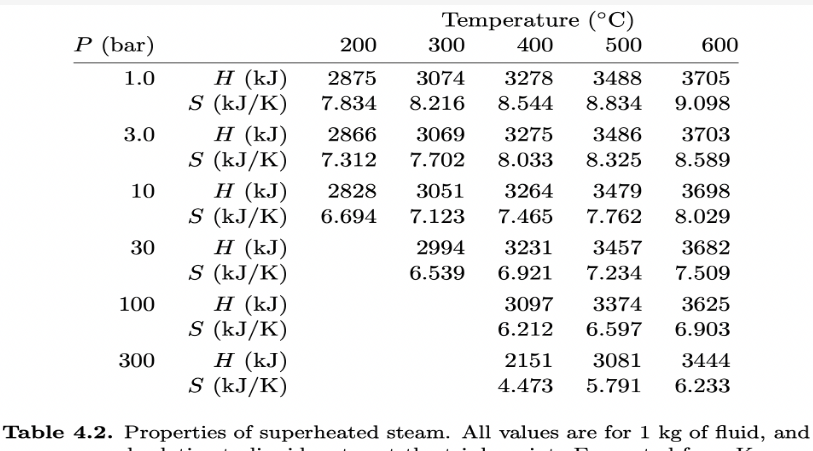


For this question, use steam tables in Schroeder's chapter 4, available here: T P H water Hsteam Swater Ssteam (C) (bar) (kJ) (kJ) (KJ/K) (KJ/K) 0 0.006 0 2501 0 9.156 10 0.012 42 2520 0.151 8.901 20 0.023 84 2538 0.297 8.667 30 0.042 126 2556 0.437 8.453 50 0.123 209 2592 0.704 8.076 100 1.013 419 2676 1.307 7.355Temperature (C) P (bar) 200 300 400 500 600 1.0 H (kJ) 2875 3074 3278 3488 3705 S (kJ/ K) 7.834 8.216 8.544 8.834 9.098 3.0 H (kJ) 2866 3069 3275 3486 3703 S (kJ/K) 7.312 7.702 8.033 8.325 8.589 10 H (kJ) 2828 3051 3264 3479 3698 S (kJ/K) 6.694 7.123 7.465 7.762 8.029 30 H (k.J) 2994 3231 3457 3682 S (kJ/ K) 6.539 6.921 7.234 7.509 100 H (kJ) 3097 3374 3625 S (kJ/ K) 6.212 6.597 6.903 300 H (kJ) 2151 3081 3444 S (kJ/K) 4.473 5.791 6.233 Table 4.2. Properties of superheated steam. All values are for 1 kg of fluid, and(a) Consider steam at 300C and '10 bars of pressure. During adiabatic expansion through a turbine, the temperature drops to 30C and some of the steam condenses. What is the pressure of the steam after it passes through the turbine, in bars? [+/ 2%] (b) What fraction (by mass) of the steam is condensed? [+/ 2%] (c) Looking at the steam table, can you throttle superheated steam to get it to cool down? No" What is the efficiency of a steam engine operating between 300C and 30C, with the maximum steam pressure of 10 bars? 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


