Question: For your Pre-Lab, redraw the structures below and think about their possible order of polarity. Be sure to consider their full Lewis structures and where
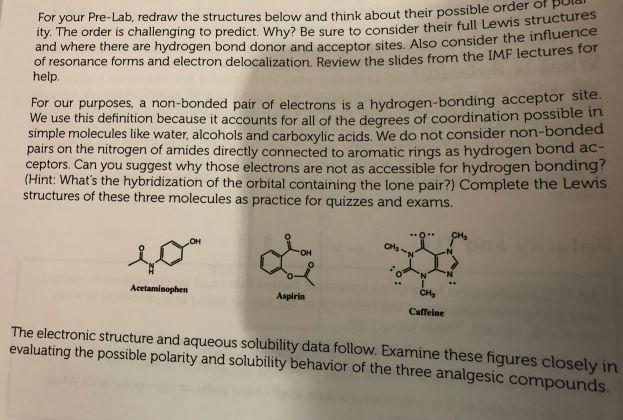
For your Pre-Lab, redraw the structures below and think about their possible order of polarity. Be sure to consider their full Lewis structures and where there are hydrogen bond donor and acceptor sites. Also consider the influence of resonance forms and electron delocalization.
For your Pre-lab, draw the structures for the solute molecules and give an educated guess their order of polarity. Provide explanation of your choices. Also, draw the structures for the solvents hexane and ethyl acetate. Which do you think is the most polar?

For your Pre-Lab, redraw the structures below and think about their possible order of phy ity. The order is challenging to predict. Why? Be sure to consider their full Lewis structures and where there are hydrogen bond donor and acceptor sites. Also consider the influence of resonance forms and electron delocalization. Review the slides from the IMF lectures for help For our purposes, a non-bonded pair of electrons is a hydrogen-bonding acceptor site We use this definition because it accounts for all of the degrees of coordination possible in simple molecules like water, alcohols and carboxylic acids. We do not consider non-bonded pairs on the nitrogen of amides directly connected to aromatic rings as hydrogen bond ac- ceptors. Can you suggest why those electrons are not as accessible for hydrogen bonding? (Hint : What's the hybridization of the orbital containing the lone pair?) Complete the Lewis structures of these three molecules as practice for quizzes and exams. OH CH Acetaminophen Aspirin CHE Caffeine The electronic structure and aqueous solubility data follow. Examine these figures closely in evaluating the possible polarity and solubility behavior of the three analgesic compounds. EXPERIMENTAL Part 1 In this part you will determine the R, values for a series of compounds using mixtures of hexane and ethyl acetate as the developing solvent. For your Pre-Lab, draw the structures for the solute molecules and give an educated guess their order of polarity. Provide some explanation of your choices. Also, draw the structures for the solvents hexane and ethyl acetate. Which do you think is the most polar? Acetophenone Anisole* Benzoic acid Butyl phenyl ether Phenol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


