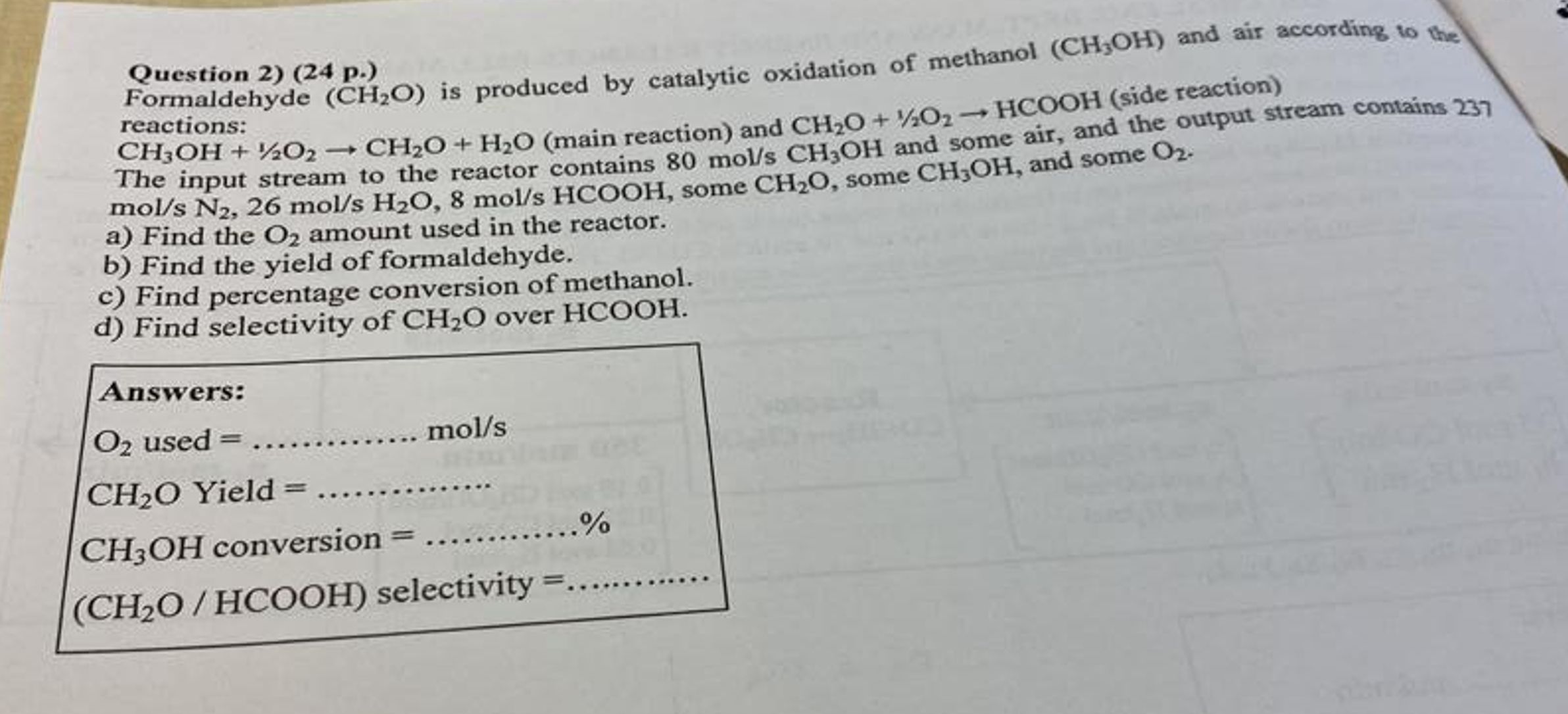
Question: Formaldehyde ( C H 2 O ) is produced by catalytic oxidation of methanol ( C H 3 O H ) and air according to
Formaldehyde is produced by catalytic oxidation of methanol and air according to the
reactions:
main reaction and side reaction
The input stream to the reactor contains and some air, and the output stream contains
some some and some
a Find the amount used in the reactor.
b Find the yield of formaldehyde.
c Find percentage conversion of methanol.
d Find selectivity of over
Formaldehyde is produced by catalytic oxidation of methanol and air according to the
reactions:
main reaction and side reaction
The input stream to the reactor contains and some air, and the output stream contains
some some and some
a Find the amount used in the reactor.
b Find the yield of formaldehyde.
c Find percentage conversion of methanol.
d Find selectivity of over
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


