Question: Formaldehyde ( H C H O ) Is produced by the catalytic reaction of methanol ( C H 3 O H ) at 7 0
Formaldehyde Is produced by the catalytic reaction of methanol at and bar according the the following reaction:
HCHO
You can assume that is independent of temperature.
If one mole of methanol is fed to the reactor, calculate the equilibrium value of
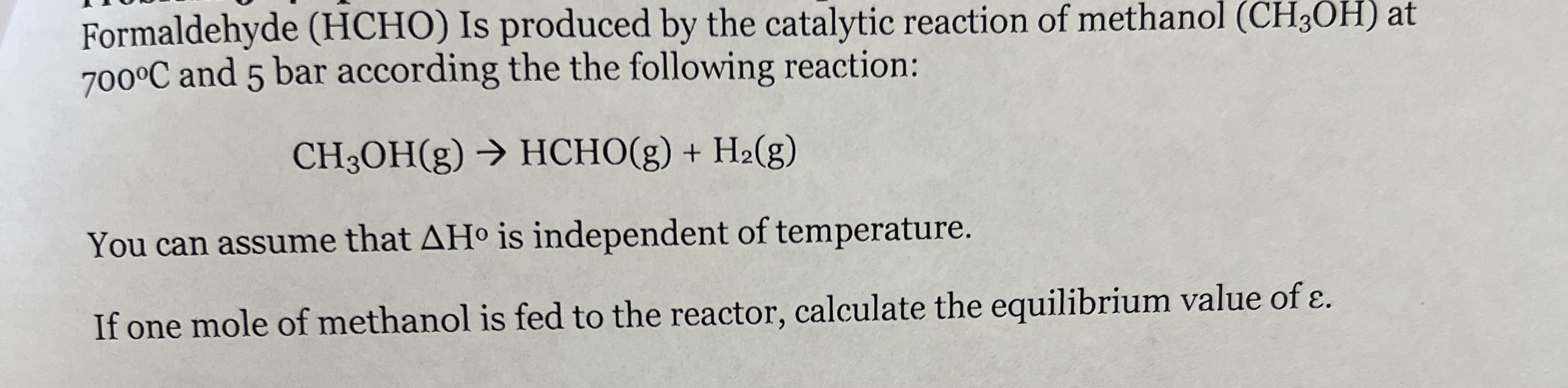
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


