Question: Formula PV = k Select the appropriate formula from the table and use it to solve for the unknown in the word problem. A gas
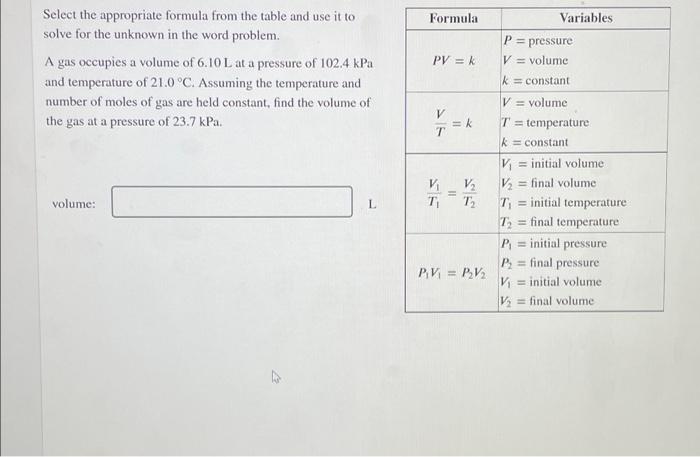
Formula PV = k Select the appropriate formula from the table and use it to solve for the unknown in the word problem. A gas occupies a volume of 6.10 L at a pressure of 102.4 kPa and temperature of 21.0 C. Assuming the temperature and number of moles of gas are held constant, find the volume of the gas at a pressure of 23.7 kPa. V =k T Variables P = pressure V = volume k = constant V = volume T = temperature k = constant Vi = initial volume V = final volume T = initial temperature T = final temperature P = initial pressure P, = final pressure V = initial volume V = final volume V T V T2 volume: L PV = P2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


