Question: Formula Total Valence Electrons Rough Lewis Structure Electron-Group Geometry/ - Include formal charges other than 0 Molecular Geometry Electron-Group Geo. TeF Molecular Geometry: Bond Polarity
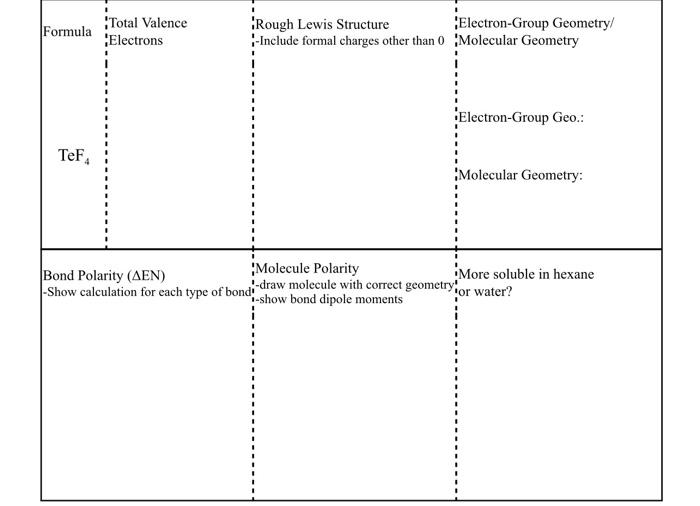
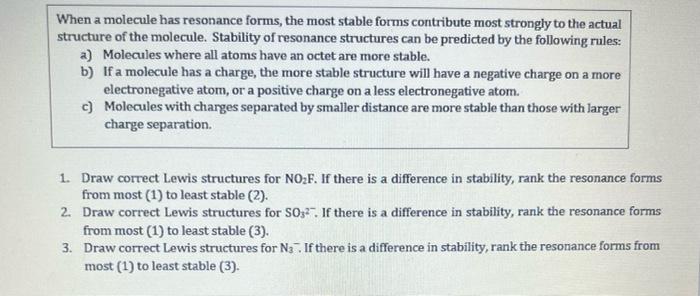
Formula Total Valence Electrons Rough Lewis Structure Electron-Group Geometry/ - Include formal charges other than 0 Molecular Geometry Electron-Group Geo. TeF Molecular Geometry: Bond Polarity (AEN) Molecule Polarity - draw molecule with correct geometry: More soluble in hexane Show calculation for each type of bond: -show bond dipole moments for water? When a molecule has resonance forms, the most stable forms contribute most strongly to the actual structure of the molecule. Stability of resonance structures can be predicted by the following rules: a) Molecules where all atoms have an octet are more stable. b) If a molecule has a charge, the more stable structure will have a negative charge on a more electronegative atom, or a positive charge on a less electronegative atom. c) Molecules with charges separated by smaller distance are more stable than those with larger charge separation 1. Draw correct Lewis structures for NOzF. If there is a difference in stability, rank the resonance forms from most (1) to least stable (2). 2. Draw correct Lewis structures for S03. If there is a difference in stability, rank the resonance forms from most (1) to least stable (3). 3. Draw correct Lewis structures for N3. If there is a difference in stability, rank the resonance forms from most (1) to least stable (3)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


