Question: Four different compounds can be formed by reacting 2 4 7 0 . 4 0 g of oxygen. a ) Calculate the mass of oxygen
Four different compounds can be formed by reacting of oxygen. a Calculate the mass of oxygen per grams o your results based on the atomic theory?
or
of a compound is combusted entirely and formed of carbon dioxide and of water. What is the empirical formula of this compound
b How can you justify
a Let's assume that you have two solutions of NaCl in two different beakers. One of them contains of of NaCl and the others has of of NaCl. After mixing these solution, the total volume is brought to by evaporation. What is the final concentration of NaCl
b A scientist would like to determine the mass percentage of chloride ion in seawater. She took sample of seawater from the sea. After the titration of seawater with silver nitrate, solid silver chloride was formed, and the volume of silver nitrate used in titration was found to be If the density of seawater is what is the mass percentage of chloride ion in the seawater?
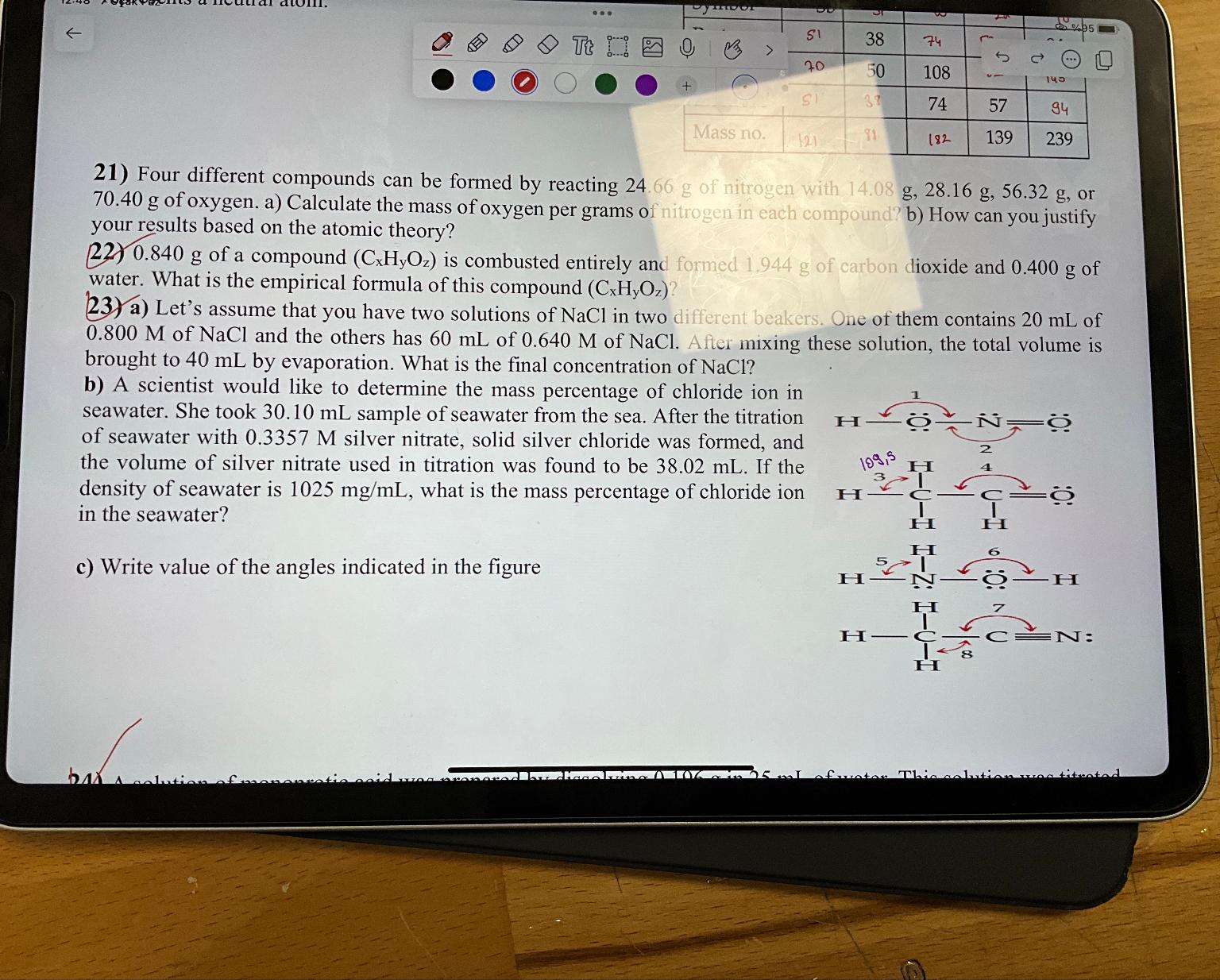
c Write value of the angles indicated in the figure
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


