Question: Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the
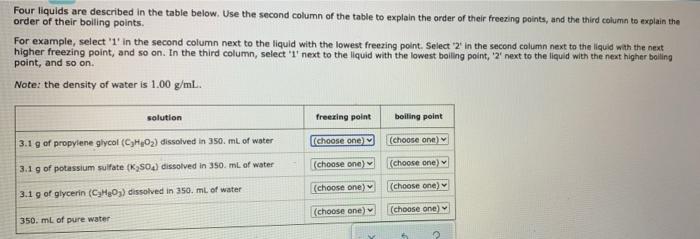
Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select 2 In the second column next to the liquid with the next higher freezing point, and so on. In the third column, select "' next to the liquid with the lowest boiling point, '2" next to the liquid with the next higher balling point, and so on. Note: the density of water is 1.00 g/mL. bolting point solution freezing point 3.1 g of propylene glycol (CO2) dissolved in 350 mL of water [choose one) (choose one) 3.1 g of potassium sulfate (K 50c) dissolved in 350 mL of water (choose one) (choose one) (choose one) (choose one) 3.1 g of glycerin (CHO) dissolved in 350. mL of water (choose one) (choose one) 350 mL of pure water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


