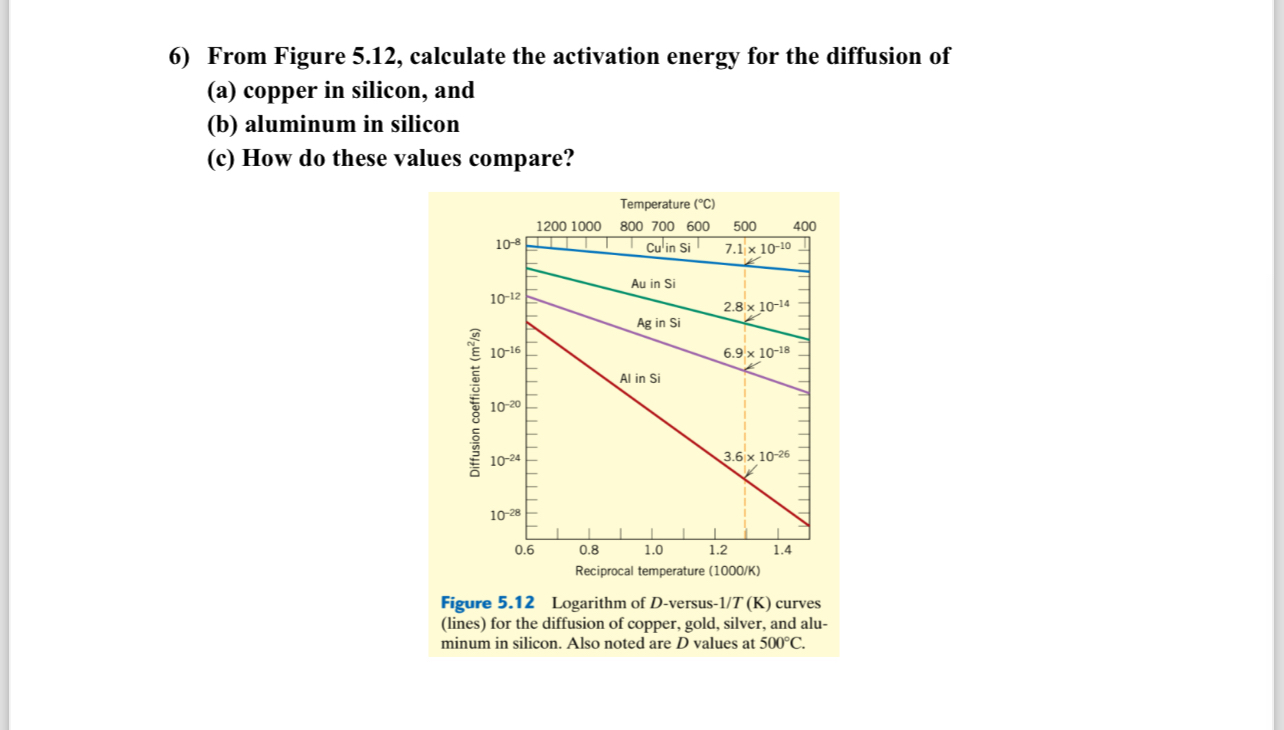
Question: From Figure 5 . 1 2 , calculate the activation energy for the diffusion of ( a ) copper in silicon, and ( b )
From Figure calculate the activation energy for the diffusion of
a copper in silicon, and
b aluminum in silicon
c How do these values compare?
Temnaration
Imes for the dirfusion of copper, gold, silver, and alu
minum in silicon. Also noted are values at
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


