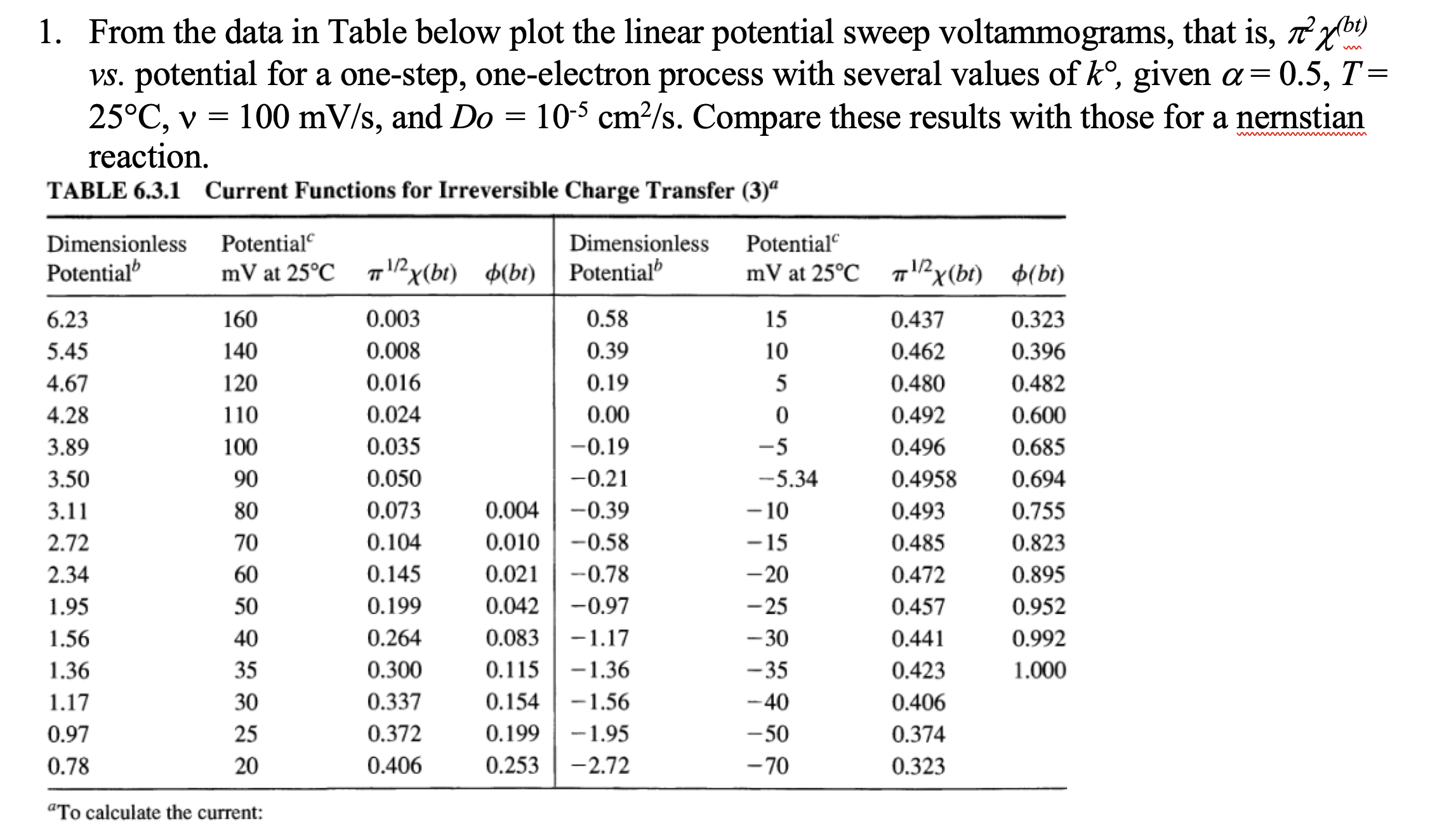
Question: From the data in Table below plot the linear potential sweep voltammograms, that is , 2 ( b t ) v s . potential for
From the data in Table below plot the linear potential sweep voltammograms, that is
potential for a onestep, oneelectron process with several values of given
and Compare these results with those for a nernstian
reaction.
TABLE Current Functions for Irreversible Charge Transfer
To calculate the current:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


