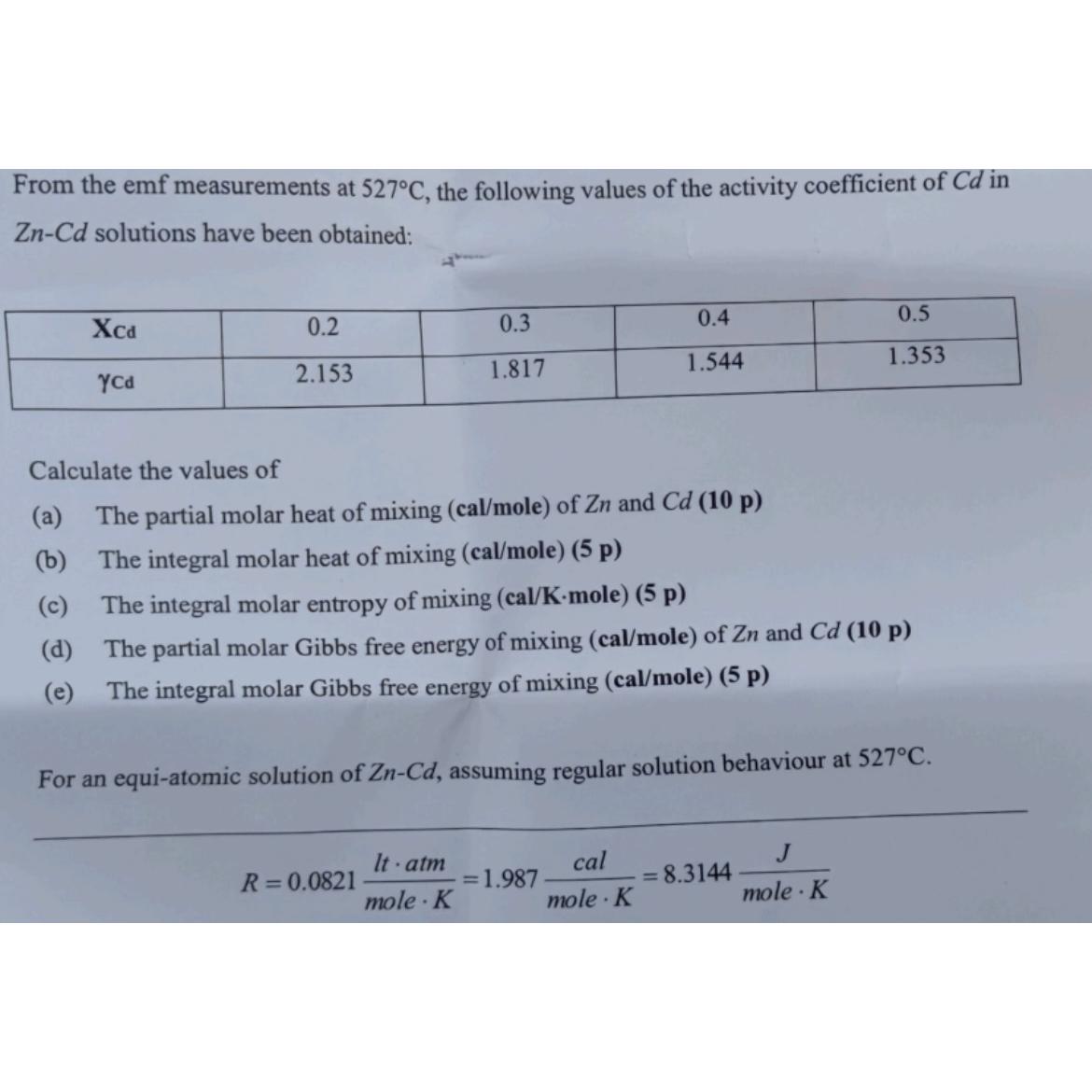
Question: From the emf measurements at 5 2 7 C , the following values of the activity coefficient of C d in Z n - C
From the emf measurements at the following values of the activity coefficient of in solutions have been obtained:
table
Calculate the values of
a The partial molar heat of mixing calmole of and
b The integral molar heat of mixing calmole
c The integral molar entropy of mixing
d The partial molar Gibbs free energy of mixing calmole of and
e The integral molar Gibbs free energy of mixing calmole p
For an equiatomic solution of assuming regular solution behaviour at
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


