Question: from this data, how do you calculate the intial concentration of 2-chloro-2mbethylpropane in the rxn mixture? what was the concentration of 2-chloro-2-methylpropane at each sampling

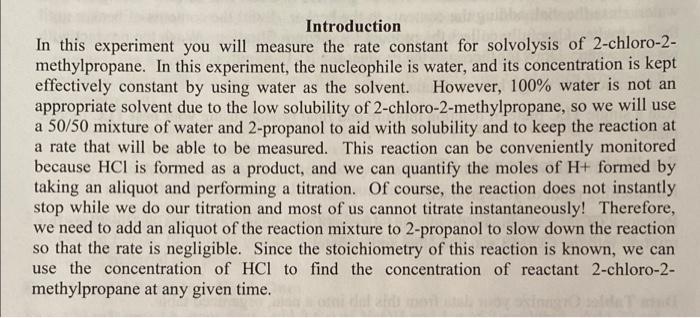
(me) (me) (ml) Time Final Buret inital Buret volume Naolt 20:03 10.2ml 6.10mL 4.01mL 30:13 21.7mL 10.2mL 11.5 mL 50:19 27.10mL 11.30mL 1:00:12 19.0 mL 3.00 ML 16.0mL 1:20.14 [13.0eme 0.0ome 15.8 m. 17.0ML Introduction In this experiment you will measure the rate constant for solvolysis of 2-chloro-2- methylpropane. In this experiment, the nucleophile is water, and its concentration is kept effectively constant by using water as the solvent. However, 100% water is not an appropriate solvent due to the low solubility of 2-chloro-2-methylpropane, so we will use a 50/50 mixture of water and 2-propanol to aid with solubility and to keep the reaction at a rate that will be able to be measured. This reaction can be conveniently monitored because HCI is formed as a product, and we can quantify the moles of H+ formed by taking an aliquot and performing a titration. Of course, the reaction does not instantly stop while we do our titration and most of us cannot titrate instantaneously! Therefore, we need to add an aliquot of the reaction mixture to 2-propanol to slow down the reaction so that the rate is negligible. Since the stoichiometry of this reaction is known, we can use the concentration of HCl to find the concentration of reactant 2-chloro-2- methylpropane at any given time
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


