Question: from this solution to the problem, how do you end up with bar at the end? what happens with the units of gravity ? what
from this solution to the problem, how do you end up with bar at the end? what happens with the units of gravity ? what unit conversion factors are been used here in order to finish with bar at the end ?
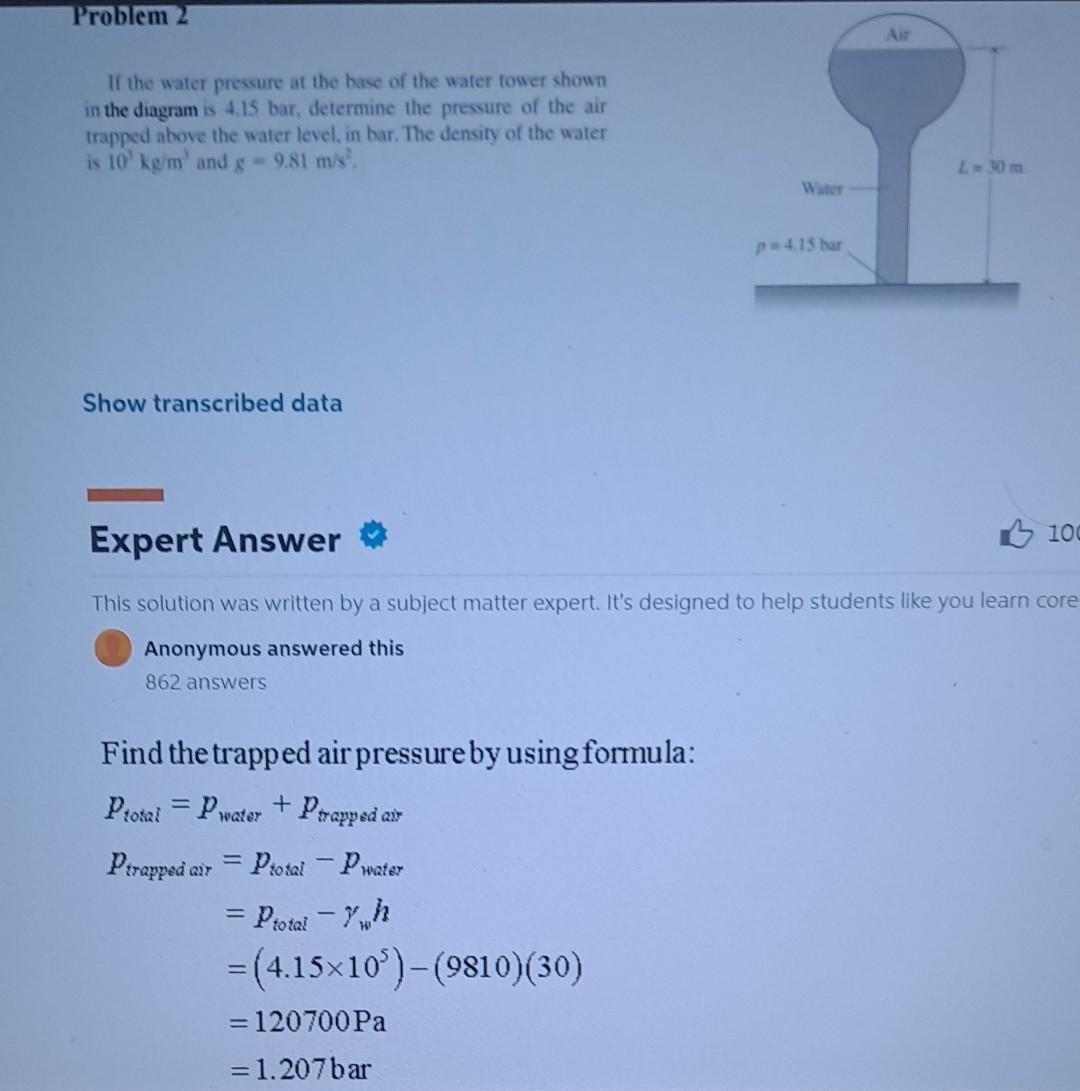
If the water pressure at the base of the water tower shown in the diagram is 4.15 bar, determine the pressure of the air trapped above the water level, in bar. The density of the water is 103kg/m3 and g=9.81m/s2. Show transcribed data Expert Answer This solution was written by a subject matter expert. It's designed to help students like you learn cor Anonymous answered this 862 answers Find the trapped air pressure by using formula: ptotal=pwater+ptrappedairptrappedair=ptotalpwater=ptotalwh=(4.15105)(9810)(30)=120700Pa=1.207bar
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


