Question: fSection Name . Convert the following units. For parts b-d, dimensional analysis MUST be used to receive credit. Some conversion factors and formulas can be
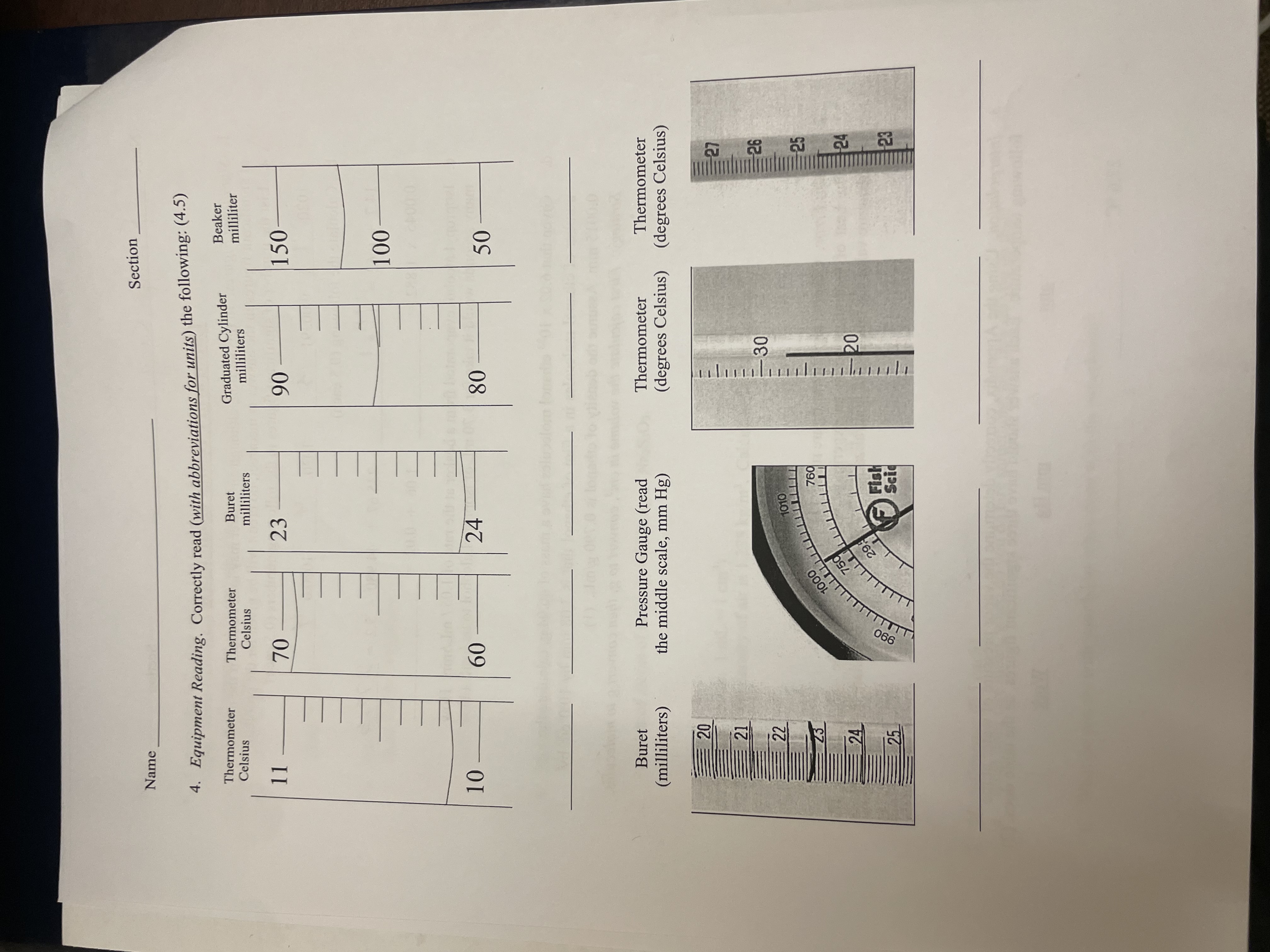


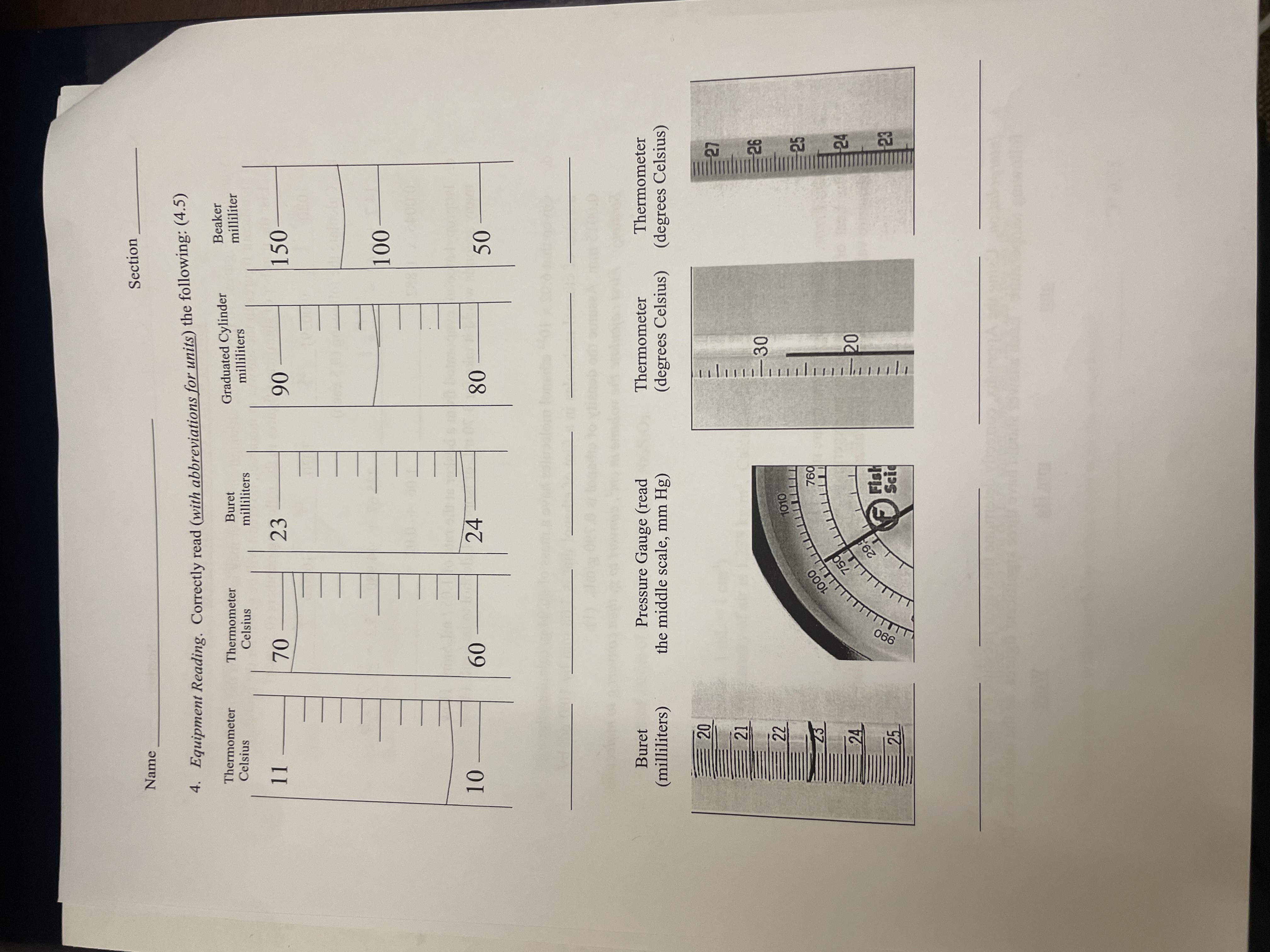


\fSection Name . Convert the following units. For parts b-d, dimensional analysis MUST be used to receive credit. Some conversion factors and formulas can be found in the Appendix. Show all work and box all answers. Observe significant figures rules. (8) a. Temperature 75.2 .C to K 23.62 .C to OF b. Pressure 29.17 inches of Hg to Pa 29.17 inches Hg to atm c. Moles, mass, and particles 53.2895 g water to moles water Number of atoms of sulfur in 0.0218 kg of Naz$203. d. Density (Note: 1 mL = 1 cm') At 1 atm, the density of air is 1.225 kg/m'. Calculate the density in g/L. 0.1265 kg gallium to mL gallium (the density of gallium is 5.91 g/cm?) Nan art 7. Miscellaneous (0.5 each) a. What does tare mean, when do you do tare something, and how do you do that? b. What do you do with your buret when done with the experiment?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


