Question: (g ( H,S() (S 8. Given the reaction SO. +2H,SE) + 256) + 2H,0 (6) 2(g) AH, kj/mol SO2() Sc) H2O(g) -296.9 -21 0 -241.8
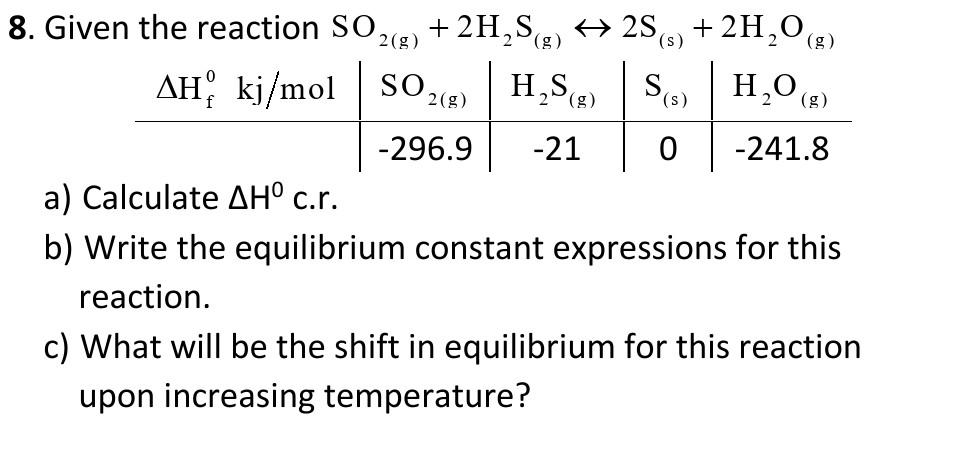
(g ( H,S() (S 8. Given the reaction SO. +2H,SE) + 256) + 2H,0 (6) 2(g) AH, kj/mol SO2() Sc) H2O(g) -296.9 -21 0 -241.8 a) Calculate AH c.r. b) Write the equilibrium constant expressions for this reaction. c) What will be the shift in equilibrium for this reaction upon increasing temperature
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


