Question: Gas A diffuses from Point 1 to a catalyst surface at Point 2, where it reacts as follows: 2A B Gas B diffuses back a
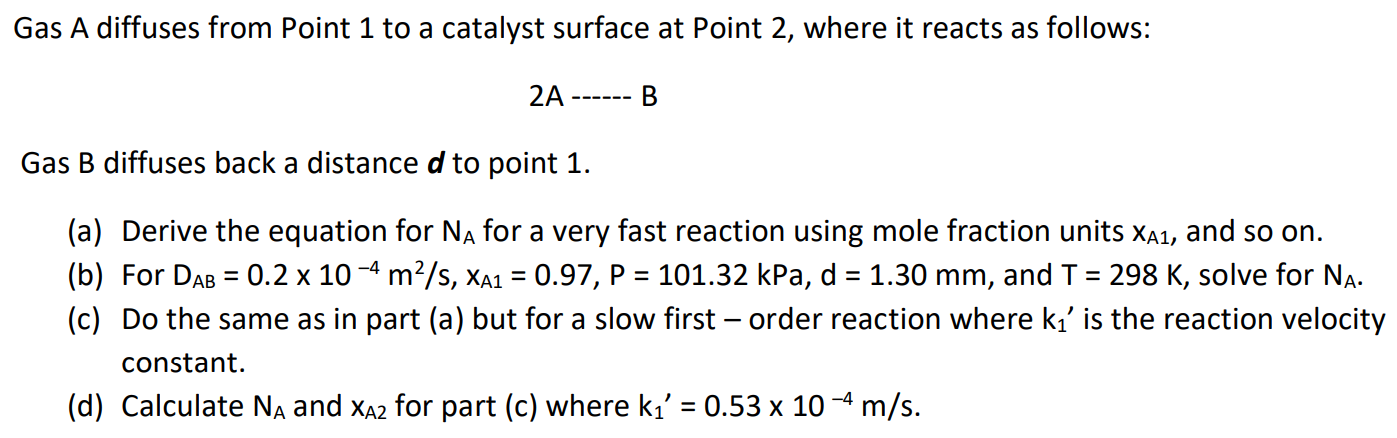
Gas A diffuses from Point 1 to a catalyst surface at Point 2, where it reacts as follows: 2A B Gas B diffuses back a distance d to point 1. = = = = = (a) Derive the equation for NA for a very fast reaction using mole fraction units Xa1, and so on. (b) For DAB = 0.2 x 10-4 m2/s, XA1 = 0.97, P = 101.32 kPa, d = 1.30 mm, and T = 298 K, solve for Na. (c) Do the same as in part (a) but for a slow first-order reaction where k' is the reaction velocity constant. (d) Calculate Na and XA2 for part (c) where k' = 0.53 x 10-4 m/s. =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


