Question: Give me complete solution, asap I'll upvote The answer should be Deviation = 0.19% Use the perfect gas equation with R=462J/kgK to compute the specific
Give me complete solution, asap I'll upvote The answer should be Deviation = 0.19%

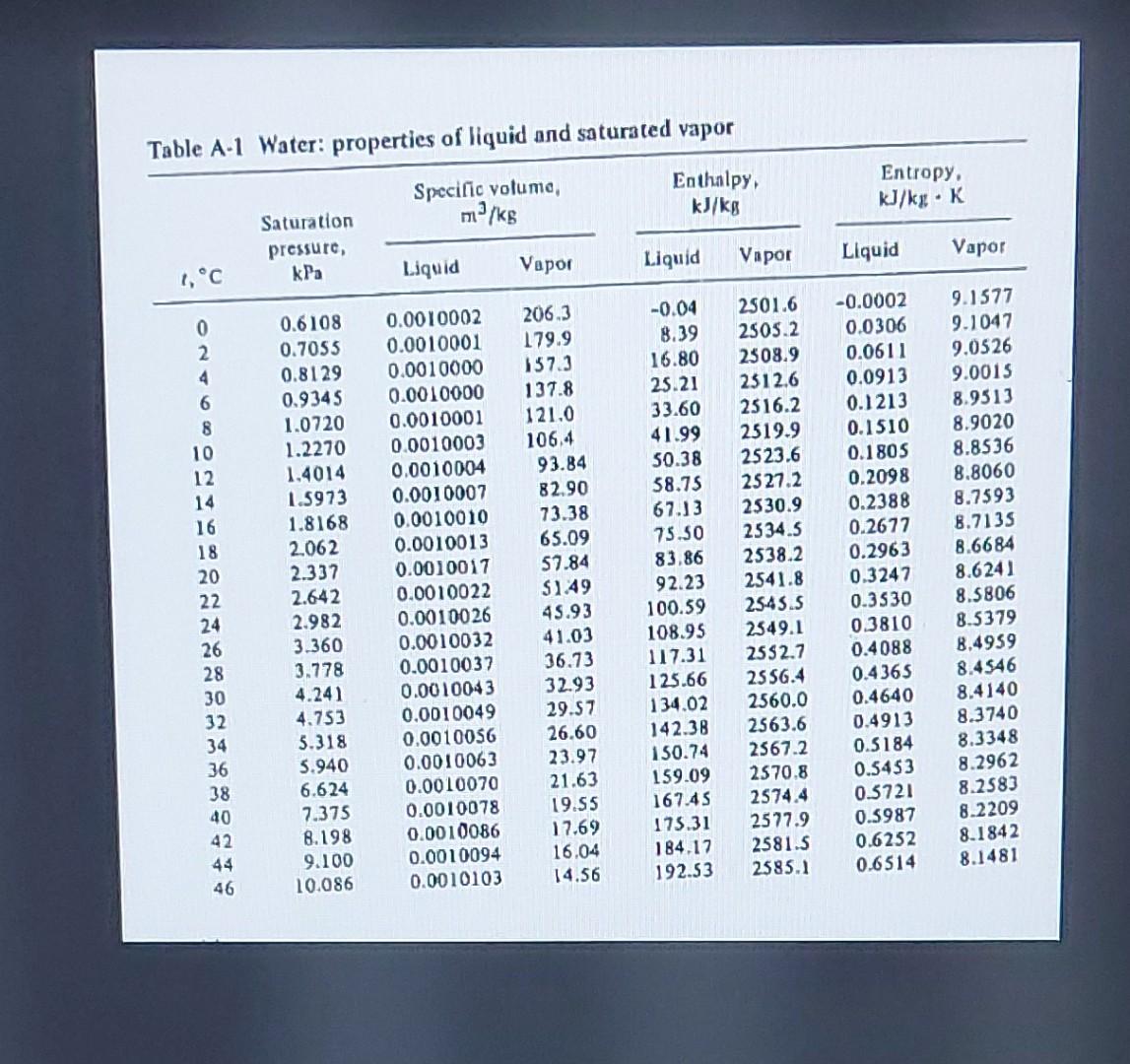
Use the perfect gas equation with R=462J/kgK to compute the specific volume of saturated vapor water at 20C. Compare with data of Table A-1. Ans. Deviation =0.19% Table A-1 Water: properties of liquid and saturated vapor \begin{tabular}{|c|c|c|c|c|c|c|c|} \hline \multirow[b]{2}{*}{1,C} & \multirow{2}{*}{\begin{tabular}{c} Saturation \\ pressure, \\ kPa \end{tabular}} & \multicolumn{2}{|c|}{\begin{tabular}{l} Specific volume, \\ m3/kg \end{tabular}} & \multicolumn{2}{|c|}{\begin{tabular}{c} Enthalpy, \\ kJ/kg \end{tabular}} & \multicolumn{2}{|c|}{\begin{tabular}{l} Entropy, \\ kJ/kgK \end{tabular}} \\ \hline & & Liquid & Vapor & Liquid & Vapor & Liquid & Vapor \\ \hline 0 & 0.6108 & 0.0010002 & 206.3 & -0.04 & 2501.6 & -0.0002 & 9.1577 \\ \hline 2 & 0.7055 & 0.0010001 & 179.9 & 8.39 & 2505.2 & 0.0306 & 9.1047 \\ \hline 4 & 0.8129 & 0.0010000 & 157.3 & 16.80 & 2508.9 & 0.0611 & 9.0526 \\ \hline 6 & 0.9345 & 0.0010000 & 137.8 & 25.21 & 2512.6 & 0.0913 & 9.0015 \\ \hline 8 & 1.0720 & 0.0010001 & 121.0 & 33.60 & 2516.2 & 0.1213 & 8.9513 \\ \hline 10 & 1.2270 & 0.0010003 & 106.4 & 41.99 & 2519.9 & 0.1510 & 8.9020 \\ \hline 12 & 1.4014 & 0.0010004 & 93.84 & 50.38 & 2523.6 & 0.1805 & 8.8536 \\ \hline 14 & 1.5973 & 0.0010007 & 82.90 & 58.75 & 2527.2 & 0.2098 & 8.8060 \\ \hline 16 & 1.8168 & 0.0010010 & 73.38 & 67.13 & 2530.9 & 0.2388 & 8.7593 \\ \hline 18 & 2.062 & 0.0010013 & 65.09 & 75.50 & 2534.5 & 0.2677 & 8.7135 \\ \hline 20 & 2.337 & 0.0010017 & 57.84 & 83,86 & 2538.2 & 0.2963 & 8.6684 \\ \hline 22 & 2.642 & 0.0010022 & $1.49 & 92.23 & 2541.8 & 0.3247 & 8.6241 \\ \hline 24 & 2.982 & 0.0010026 & 45.93 & 100.59 & 25.45 .5 & 0.3530 & 8.5806 \\ \hline 26 & 3.360 & 0.0010032 & 41.03 & 108.95 & 2549.1 & 0.3810 & 8.5379 \\ \hline 28 & 3.778 & 0.0010037 & 36.73 & 117.31 & 2552.7 & 0.4088 & 8,4959 \\ \hline 30 & 4.241 & 0.0010043 & 32.93 & 125.66 & 2556.4 & 0.4365 & 8.4546 \\ \hline 32 & 4.753 & 0.0010049 & 29.57 & 134.02 & 2560.0 & 0.4640 & 8.4140 \\ \hline 34 & 5.318 & 0.0010056 & 26.60 & 142.38 & 2563.6 & 0.4913 & 8.3740 \\ \hline 36 & 5.940 & 0.0010063 & 23.97 & 150.74 & 2567.2 & 0.5184 & 8.3348 \\ \hline 38 & 6.624 & 0.0010070 & 21.63 & 159.09 & 2570.8 & 0.5453 & 8.2962 \\ \hline 40 & 7.375 & 0.0010078 & 19.55 & 167.45 & 2574.4 & 0.5721 & 8.2583 \\ \hline 42 & 8.198 & 0.0010086 & 17.69 & 175.31 & 2577.9 & 0.5987 & 8.2209 \\ \hline 44 & 9.100 & 0.0010094 & 16.04 & 184.17 & 2581.5 & 0.6252 & 8.1842 \\ \hline 46 & 10.086 & 0.0010103 & 14.56 & 192.53 & 2585.1 & 0.6514 & 8.1481 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


