Question: Q. 1. (15) Some of the energy levels of a hypothetical one electron atom (not hydrogen) are listed in the table below. 1 3
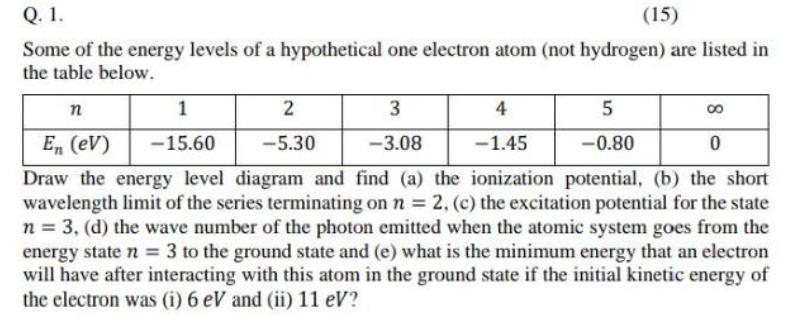
Q. 1. (15) Some of the energy levels of a hypothetical one electron atom (not hydrogen) are listed in the table below. 1 3 4 5 En (eV) -15.60 -5.30 -3.08 -1.45 -0.80 Draw the energy level diagram and find (a) the ionization potential, (b) the short wavelength limit of the series terminating on n = 2, (c) the excitation potential for the state n = 3, (d) the wave number of the photon emitted when the atomic system goes from the energy state n = 3 to the ground state and (e) what is the minimum energy that an electron will have after interacting with this atom in the ground state if the initial kinetic energy of the electron was (i) 6 eV and (ii) 11 eV?
Step by Step Solution
3.34 Rating (163 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
60d452b08681a_227957.pdf
180 KBs PDF File
60d452b08681a_227957.docx
120 KBs Word File


