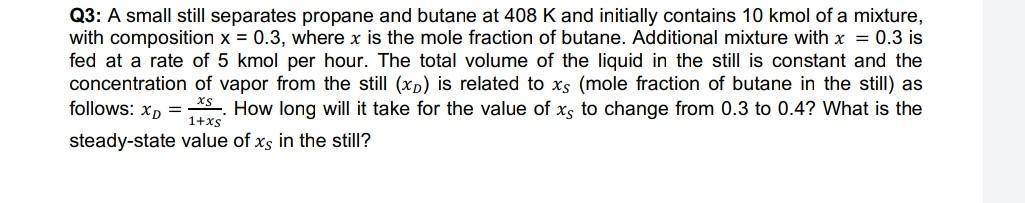
Question: give the solution by explaining the terms Q3: A small still separates propane and butane at 408 K and initially contains 10 kmol of a
give the solution by explaining the terms
Q3: A small still separates propane and butane at 408 K and initially contains 10 kmol of a mixture, with composition x = 0.3, where x is the mole fraction of butane. Additional mixture with x = 0.3 is fed at a rate of 5 kmol per hour. The total volume of the liquid in the still is constant and the concentration of vapor from the still (xp) is related to xs (mole fraction of butane in the still as follows: x) = *S. How long will it take for the value of xs to change from 0.3 to 0.4? What is the 1+xs steady-state value of xs in the still
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


