Question: Given a 54.6-g sample of this substance with a specific heat of 51.3 J/(kgC), how much heat is required to change its temperature from 180.0
 Given a 54.6-g sample of this substance with a specific heat of 51.3 J/(kgC), how much heat is required to change its temperature from 180.0 C to 238.0 C?
Given a 54.6-g sample of this substance with a specific heat of 51.3 J/(kgC), how much heat is required to change its temperature from 180.0 C to 238.0 C?
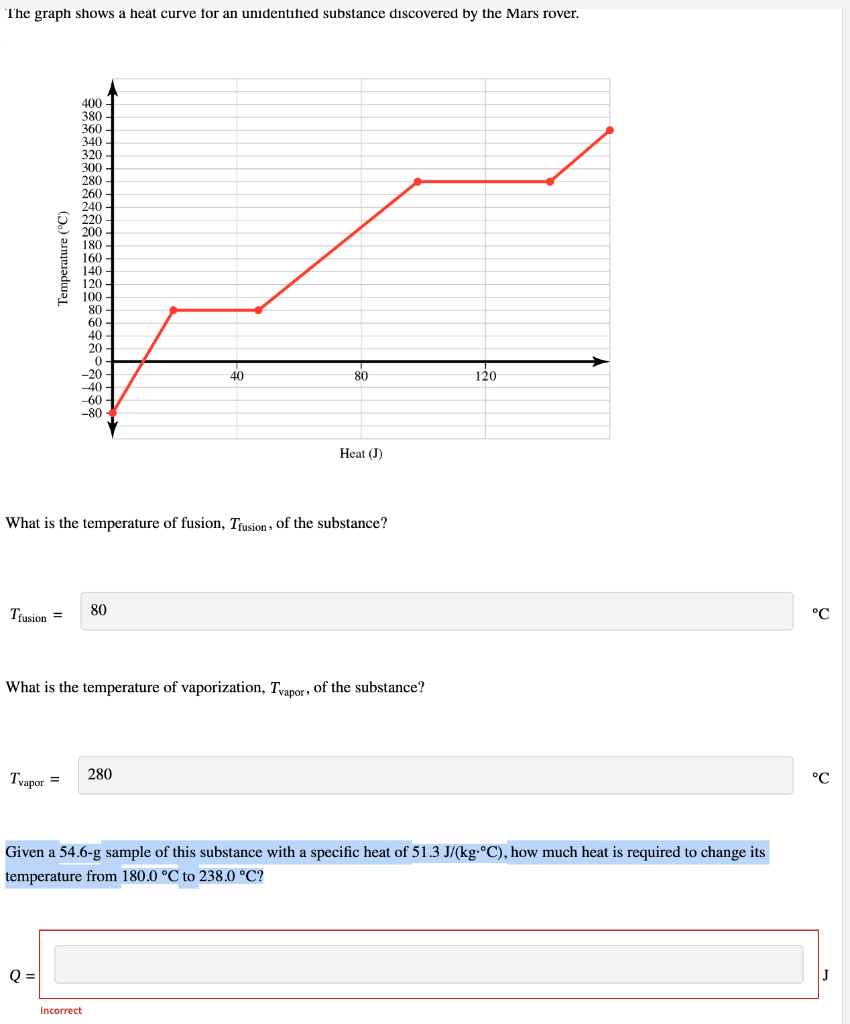
The graph shows a heat curve tor an unidentined substance discovered by the Mars rover. What is the temperature of fusion, Tfusion, of the substance? Tfusion= What is the temperature of vaporization, Tvapor, of the substance? Tvapor= Given a 54.6-g sample of this substance with a specific heat of 51.3J/(kgC), how much heat is required to change its temperature from 180.0C to 238.0C ? Q=
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


