Question: Given liquid-vapor equilibrium data for fluid A and fluid B at 332.99 K below, Pressure, 1.2717 1.2946 1.3196 1.3332 1.3749 1.3988 1.4119 1.4230 bar 0.0000
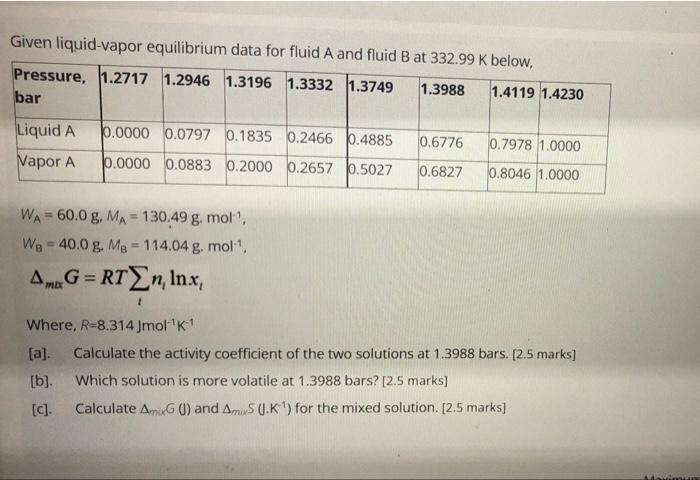
Given liquid-vapor equilibrium data for fluid A and fluid B at 332.99 K below, Pressure, 1.2717 1.2946 1.3196 1.3332 1.3749 1.3988 1.4119 1.4230 bar 0.0000 0.0797 0.1835 0.2466 0.4885 Liquid A Vapor A 0.6776 0.7978 1.0000 0.0000 0.0883 0.2000 0.2657 0.5027 0.6827 0.8046 1.0000 WA= 60.0 g, MA= 130,49 g.mol, We - 40.0 g. Mg = 114.04 g. moll Am.G=RTXn, Inx; 1 Where, R=8.314 Jmol K1 [a] Calculate the activity coefficient of the two solutions at 1.3988 bars. [2.5 marks] [b] Which solution is more volatile at 1.3988 bars? [2.5 marks] [C]. Calculate Amix) and Amis O.K ') for the mixed solution. [2.5 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


