Question: Given that ko for Oxygen is 0.5, ko for Boron is 0.8 and ko for Gold is 2.5 x 10^-5. Essentially, the code in matlab
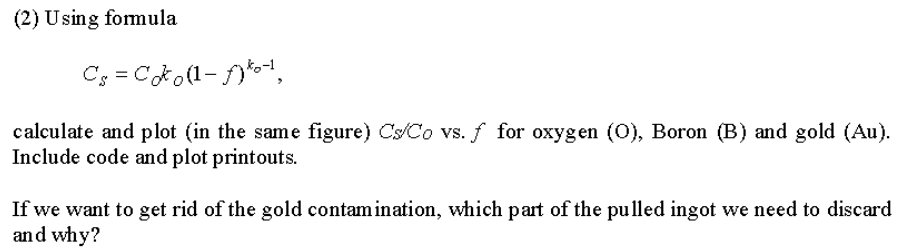
Given that ko for Oxygen is 0.5, ko for Boron is 0.8 and ko for Gold is 2.5 x 10^-5. Essentially, the code in matlab to plot k0(1-f)^ko-1 vs f is needed for any values of f.
(2) Using formula calculate and plot (in the same figure) CsCo vs. f for oxygen (O), Boron (B) and gold (Au). Include code and plot printouts. If we want to get rid of the gold contamination, which part of the pulled ingot we need to discard and why
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


