Question: Given that the c/a ratio in a hexagonal close-packed (HCP) unit cell is 1.633, please calculate the packing factor for an HCP crystal structure. (10
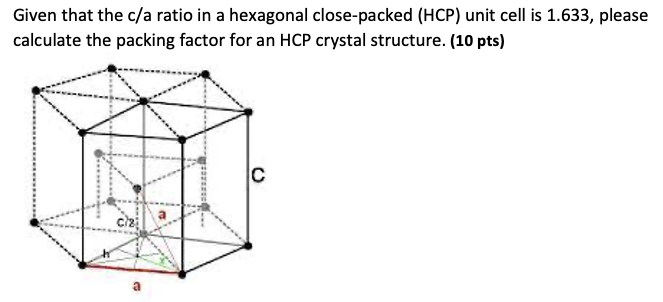
Given that the c/a ratio in a hexagonal close-packed (HCP) unit cell is 1.633, please calculate the packing factor for an HCP crystal structure. (10 pts) Cl2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


