Question: Given that the equilibrium constant for reaction (1) below is 2.85102, what is the equilibrium constant of reaction (2) under the same conditions? (1) 1/2A2(g)+1/2B2(g)AB(g)
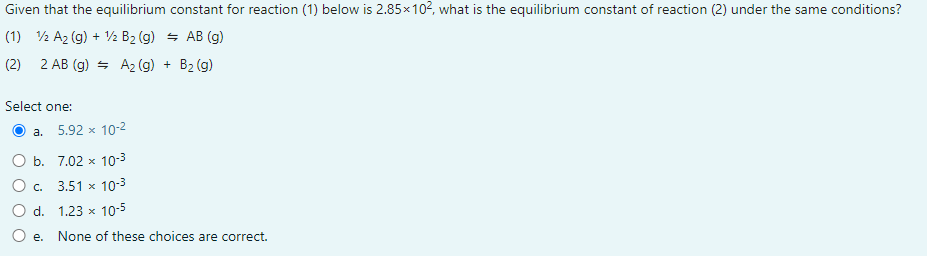
Given that the equilibrium constant for reaction (1) below is 2.85102, what is the equilibrium constant of reaction (2) under the same conditions? (1) 1/2A2(g)+1/2B2(g)AB(g) (2) 2AB(g)A2(g)+B2(g) Select one: a. 5.92102 b. 7.02103 c. 3.51103 d. 1.23105 e. None of these choices are correct
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


