Question: Given the data below (taken from standard data tables), calculate the crystal lattice energy for sodium oxide: (You will be posting your Born-Haber cycle to
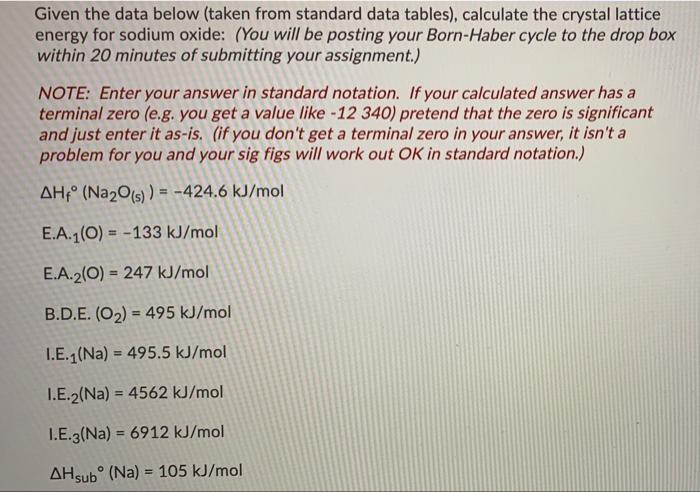
Given the data below (taken from standard data tables), calculate the crystal lattice energy for sodium oxide: (You will be posting your Born-Haber cycle to the drop box within 20 minutes of submitting your assignment.) NOTE: Enter your answer in standard notation. If your calculated answer has a terminal zero (e.g. you get a value like -12 340) pretend that the zero is significant and just enter it as-is. (if you don't get a terminal zero in your answer, it isn't a problem for you and your sig figs will work out OK in standard notation.) AH (Na2O(s)) = -424.6 kJ/mol E.A.1(O) = -133 kJ/mol E.A.2(0) = 247 kJ/mol B.D.E. (O2) = 495 kJ/mol I.E.1 (Na) = 495.5 kJ/mol I.E.2(Na) = 4562 kJ/mol I.E.3(Na) = 6912 kJ/mol AHsub (Na) = 105 kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


