Question: Given the data in the table below, calculate AH (kJ) for the reaction: CH3OH) + 3/2 029) - CO2(g) + 2H20m) Substance AHr(kJ/mol) CH3OH) -259
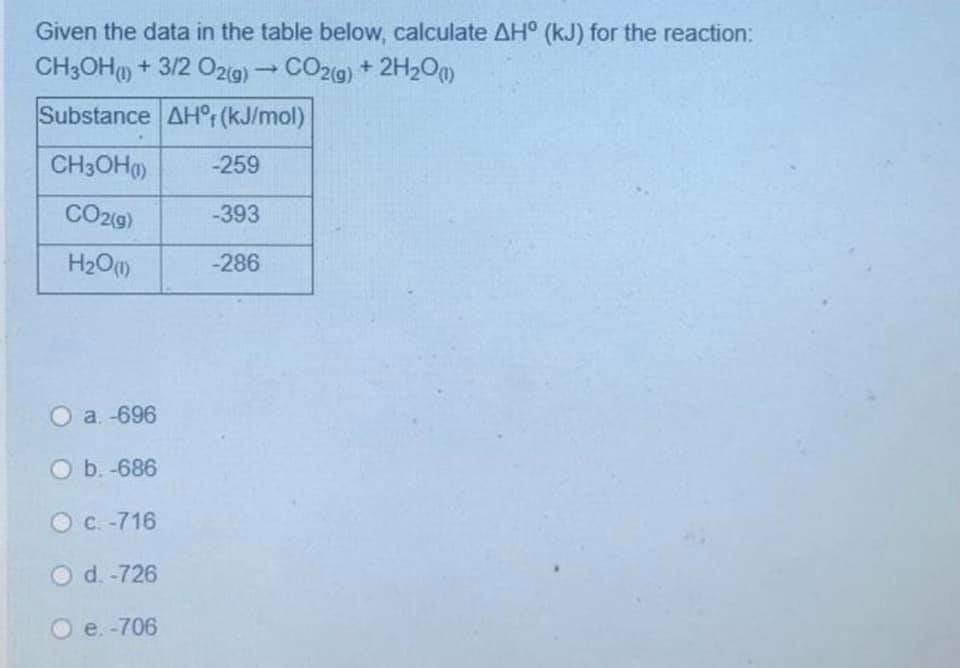
Given the data in the table below, calculate AH (kJ) for the reaction: CH3OH) + 3/2 029) - CO2(g) + 2H20m) Substance AHr(kJ/mol) CH3OH) -259 CO29) -393 H20 (1) -286 a 696 O b. -686 C-716 d. -726 e. -706
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


