Question: Given the following information: Answer the following question: Using this indicator, we will attempt to determine the concentration of various acids depending on the amount
Given the following information:
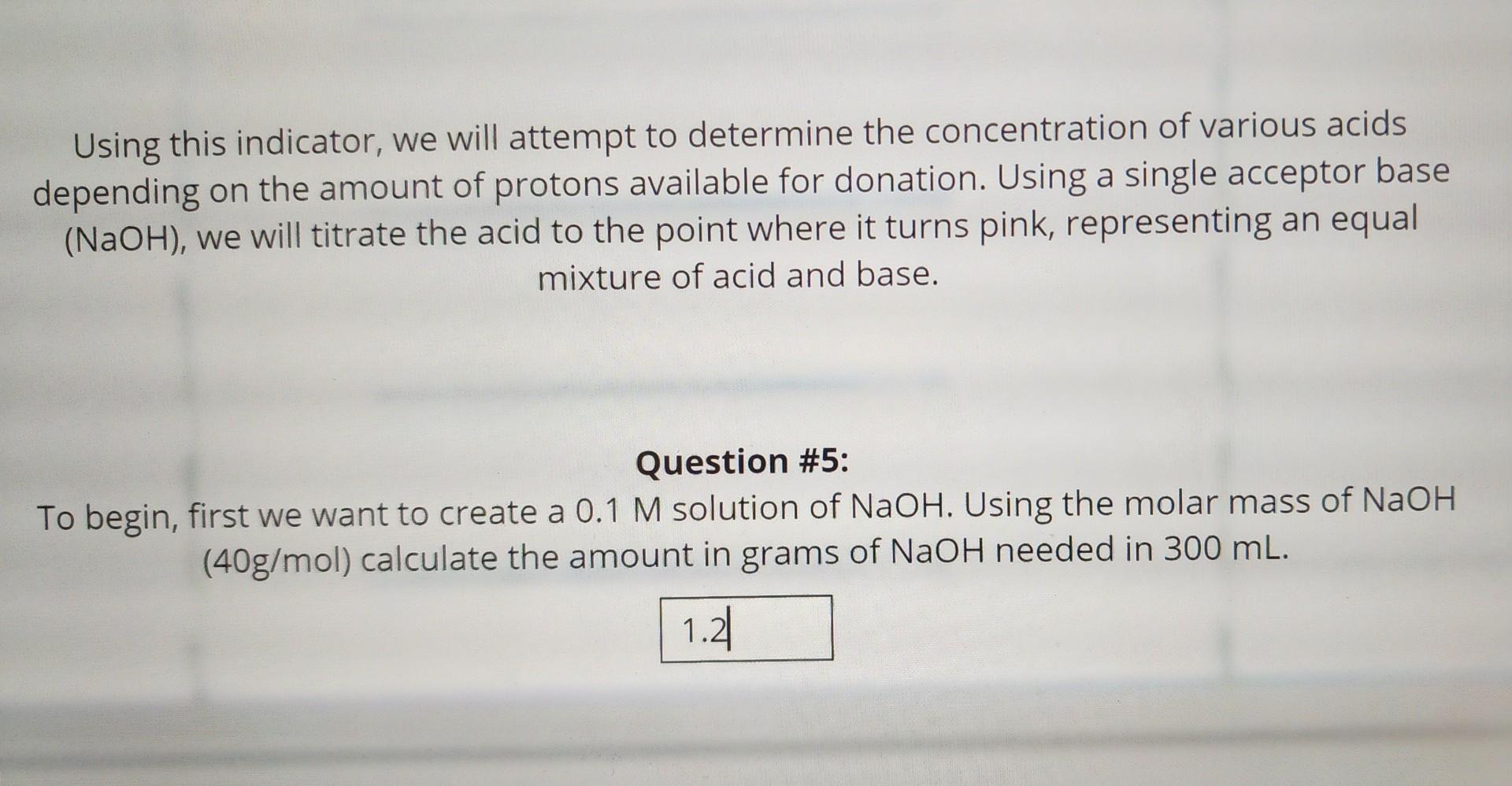




Answer the following question:

Using this indicator, we will attempt to determine the concentration of various acids depending on the amount of protons available for donation. Using a single acceptor base (NaOH), we will titrate the acid to the point where it turns pink, representing an equal mixture of acid and base. Question \#5: To begin, first we want to create a 0.1M solution of NaOH. Using the molar mass of NaOH (40g/mol) calculate the amount in grams of NaOH needed in 300mL. Using our 0.1MNaOH solution, we will prepare to titrate an acid. Before doing so we must first add phenolphthalein to our acid (this has been done for you in the following simulation). This compound acts as an indicator that is highly responsive to pH changes. With this solution of NaOH we will now add dropwise to the acid containing phenolphthalein. Beginning with your 50mL solution of acid HCl, begin to titrate in your 0.1M solution of NaOH slowly. When the solution turns pink, stop and record the amount of NaOH used. To begin the procedure click next 15mL 18.5mL Question \#6: ing the amount of Base used in step one, determine the oncentration of the acid, reference the equation below. Assume that the acid contains only one proton donor. HCl(aq)+NaOH(aq)H2O(l)+Na++ClH++OHH2O(l) Singly protic acid MacidVacid=MbaseVbase
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


