Question: Given the half-reactions and their respective standard reduction potentials 1. Cu3++2eCu+E10=+1.28V 2. Cu2+eCu+E2=+0.15V 3. Cu2++2eCu(s)E3=+0.34V 4. Cu++eCu(s)E4=+0.52V calculate the standard reduction potential for the reduction
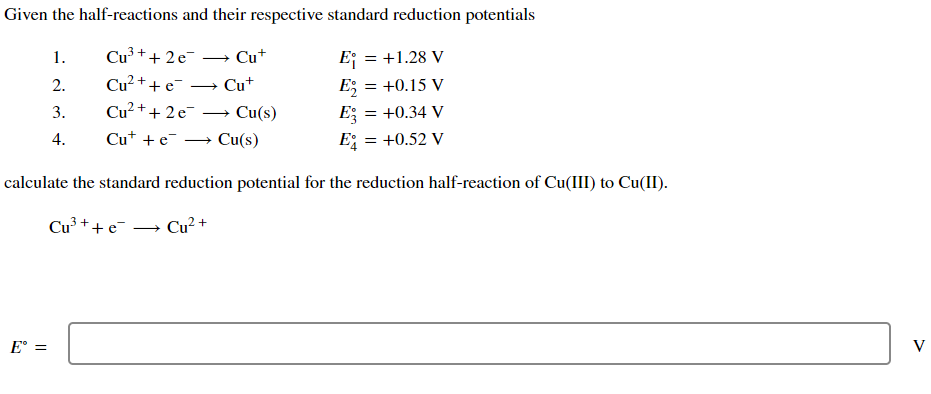
Given the half-reactions and their respective standard reduction potentials 1. Cu3++2eCu+E10=+1.28V 2. Cu2+eCu+E2=+0.15V 3. Cu2++2eCu(s)E3=+0.34V 4. Cu++eCu(s)E4=+0.52V calculate the standard reduction potential for the reduction half-reaction of Cu (III) to Cu (II). Cu3++eCu2+ E=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


