Question: Given the reaction A -B, r= k C ?, with k = 0.5 liters/mole-min and CAO = 2.0 moles/liter, and v = 4 liters/min, what
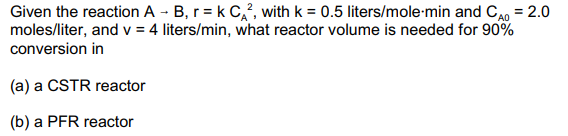
Given the reaction A -B, r= k C ?, with k = 0.5 liters/mole-min and CAO = 2.0 moles/liter, and v = 4 liters/min, what reactor volume is needed for 90% conversion in (a) a CSTR reactor (b) a PFR reactor
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


